Biomedical Engineering Reference
In-Depth Information
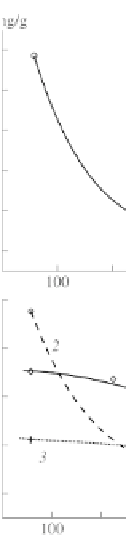
Figure 4.5
Isobars of adsorption of (1, 1', 2) oxygen and of (3) nitrogen on
(a) activated carbon and (b) fullerene (1', 3) and astralene (2)
at
p
= 200 torr.
g
The oxygen sorption isobars on activated carbon, fullerene and
astralene and the nitrogen sorption isobar on fullerene (all measured
at 200 torr) are shown in Fig. 4.5. We can clearly see that, for all
the three samples with a temperature decrease, in the beginning
a characteristic exponential increase in sorption capability is
observed.
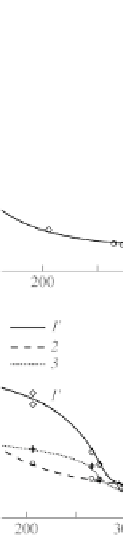
The exponential increase on temperature decreasing remains
throughout the whole temperature range for astralene and activated
carbon. At the same time, the O
sorption isobars on fullerene
deviate from the exponential dependence, demonstrating a clear
limitation of sorption value with decreasing sample temperature.
These findings lead to the conclusion that for fullerene we are in
the presence of a nonequilibrium adsorption regime, characteristic
for a wide range of adsorbents that exhibit a molecular sieves effect.
This effect has been already noted for fullerenes [22]. Under these
and N
2
2