Biomedical Engineering Reference
In-Depth Information
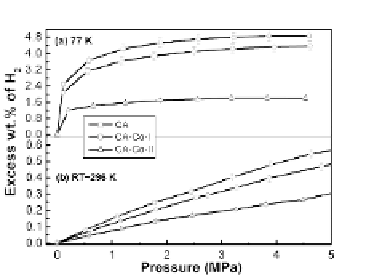
of hydrogen uptake (4.38 wt.%) at 77 K and 4.6 MPa. The measured
excess wt.% of hydrogen is much higher than that of metal-doped
CAs previously presented in the literature (2.1 wt.% for CoCA
and 2.3 wt.% for Ni-CA) [5]. The higher hydrogen storage capacity
in the Co-CA-I is attributed to the higher surface area and large
micropore volume obtained by our efficient synthesis method,
although the hydrogen uptake in CA-Co-I is approximately 10% lower
than the uptake in pure CA as shown in Table 3.6. This phenomenon
is mainly related to the decrease in micropore volume in the cobalt-
doped sample, i.e., from 0.7 cm
3
3
/g for CA-Co-I.
There is a large decrease (~67%) in hydrogen uptake for CA-Co-II
because of the decrease in surface area (77%) and micropore
volume (71%). This is because the potential field generated by
the micropore walls acts to enhance the binding energy between
hydrogen molecules and carbon adsorbent. The decrease in the
pore volume suggests that metallic particles are filling the larger
mesopores which leads to a drop in the total pore volume from
1.32 cm
/g for CA to 0.6 cm
3
3
/g for the CA-Co-I and a lower
micropore volume. This is consistent with the TEM results which
show particle sizes larger than 2 nm. It is worth pointing out that
the micropore volume of the CA-Co-II is lower than that of the CA-
Co-I, although the total pore volume is actually larger than that of
CA-Co-I. It is therefore suggested that the micropore volume plays
a more important role in hydrogen sorption than the total pore
volume.
/g for pure CA to 0.70 cm
Figure 3.12
Hydrogen sorption isotherms of CA, CA-Co-I, and CA-Co-II at
(a) 77 K and (b) room temperature (296 K).
The hydrogen uptake per unit surface area of CA is similar to
that for activated carbons, i.e., 1 wt.% for every 500 m
2
/g of surface