Biomedical Engineering Reference
In-Depth Information
Kp
Kp
(3.1)
q
1
where
is expressed as
the ratio of occupied adsorption sites to the total number of sites.
As shown in Fig. 3.1, the amount of hydrogen adsorbed
increases with decreasing temperature and increasing pressure.
With increasing pressure, hydrogen adsorption increases linearly up
to a certain pressure, but at higher pressures the increase becomes
gradual and eventually levels off at very high pressures.
K
is the adsorption-equilibrium constant and
θ
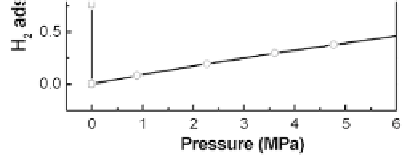
Figure 3.1
Hydrogen storage capacity of typical CA derived from
resorcinol-furfural. The measurements were conducted
at (a) liquid nitrogen temperature (77 K) and (b) at room
temperature (RT) after carbonizing at 900°C for 3 h and
activating at 900°C for 1 h.
The hydrogen sorption at room temperature for CAs is less
than 0.5 wt.% (for pressures below 6 MPa), as shown in Fig. 3.1b.
The hydrogen storage at room temperature is proportional to the
pressure, i.e., it obeys Henry's law. The results are consistent with the
hydrogen sorption for a range of nano-structured carbon materials,
which show less than 1 wt.% H
absorption at room temperature for
2
pressures below 10 MPa [16].
3.2.1.1
The Enthalpy of Adsorption
The enthalpy of adsorption (Δ
H
) can be obtained by
ads
d
ln
p
(3.2)
2
H
RT
ads
dT
q