Biomedical Engineering Reference
In-Depth Information
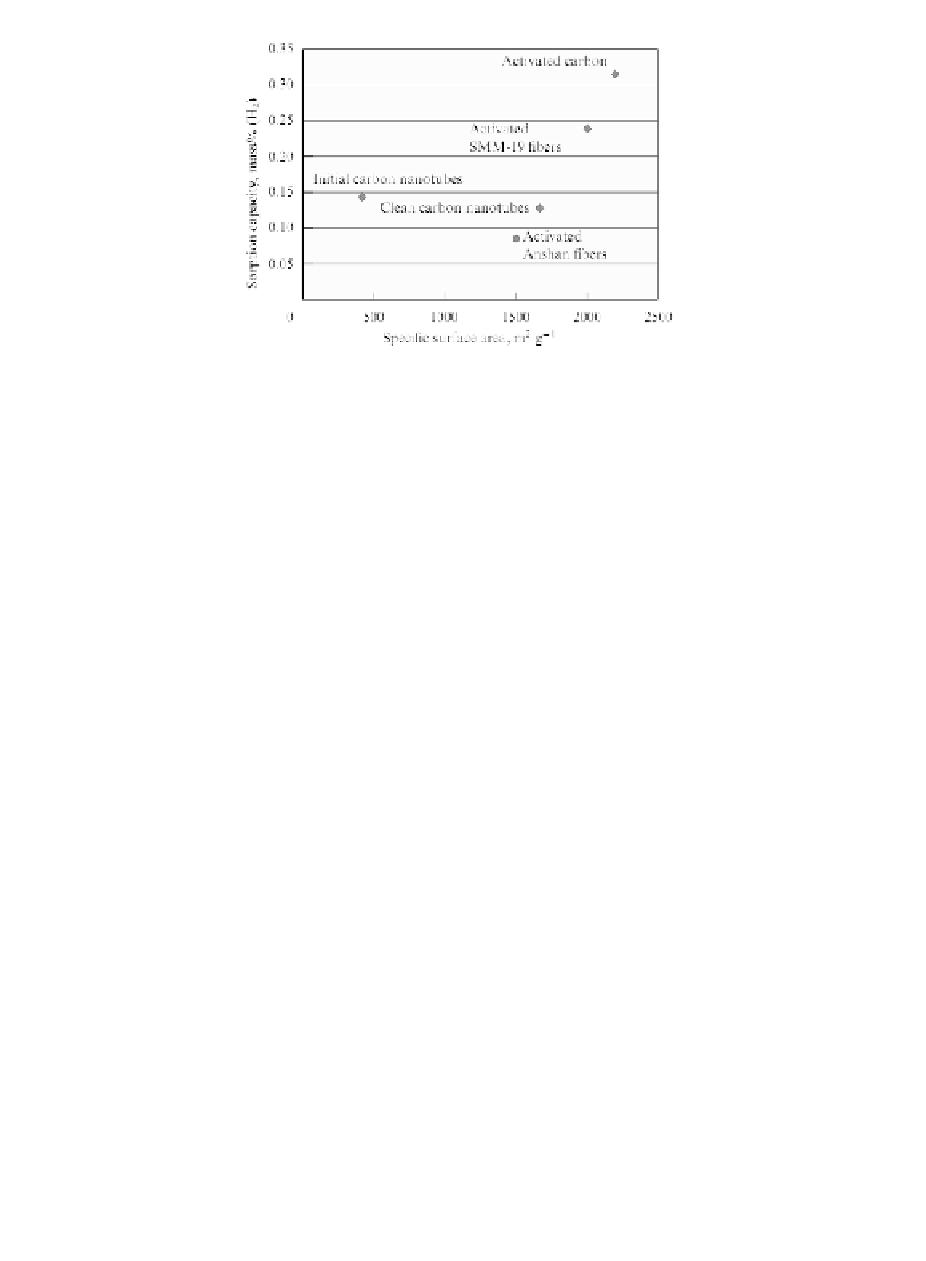
Figure 2.14
Correlation between experimental values of the specific
surface area of carbon adsorbent materials and hydrogen
sorption at room temperature and 2 MPa [73].
In the untreated carbon nanotubes described in Ref. [73], the
equilibrium related to the sorption process does not have enough
time to be settled, revealing a relatively slow (probably diffusion)
kinetics, characteristic of a certain type of hydrogen chemisorption.
This may be caused by the presence of a metallic catalyst or other
contaminants.
The isotherm of hydrogen adsorption by untreated single-wall
nanotubes [73] at about 290 K and at pressures up to 2 MPa (see
Fig. 2.13) coincides (as regards to the maximum saturation) with the
hydrogen adsorption isotherm by very “dirty” single-wall nanotube
samples Ref. [26] (Fig. 2.9b). It may be caused by the presence,
distribution and state of contaminants in the samples [73, 26].
For the single-wall nanotubes [76], it has been found that the
values of isosteric enthalpy of hydrogen adsorption at about 35 K
(∆
H
ads
) should be in the range from −7.5 to −2.5 kJ mol
−1
(H
)
2
−3
for adsorbate concentrations H
.
These values have been compared with the experimental data for
the enthalpy of hydrogen adsorption on the graphene surface of
graphite [∆
/C in the range (1.2-48)
⋅
10
2
ads
-1
)] and the enthalpy of liquefaction
(condensation) of gaseous hydrogen at 20 K [∆
H
≈ -4.2 kJ mol
(H
gr
2
H
liq
≈ -0.9 kJ mol
-1
(H
)]. Similar values of the sorption characteristics have been
obtained in Refs. [81, 82].
The isotherms of hydrogen adsorption by multiwall nanotube
samples (
2
exp
2
−1
exp
S
≈ 137 m
g
) and activated carbon AKh-21 (
S