Biomedical Engineering Reference
In-Depth Information
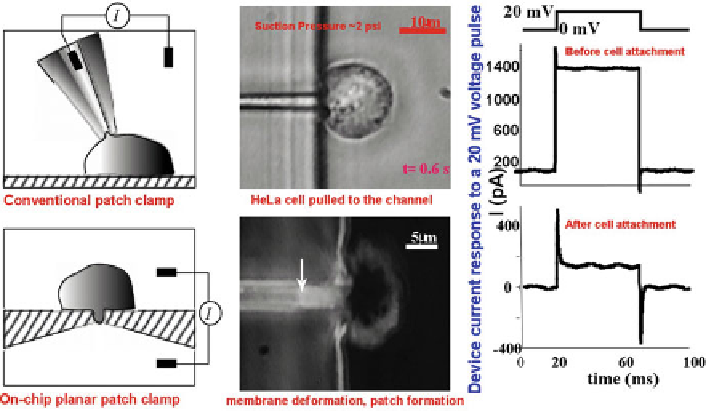
Fig. 13
Schematic drawing of a conventional patch clamp and an on-chip patch clamp (
left
). Cells
can be pulled into one of the on-chip patch pores (
top middle
), resulting in membrane deformation
and patch formation (
bottom middle
). Resulting current measured before (
top right
) and after cell
attachment (
bottom right
). For details, see Seo et al. (
2004
)
Other parallel readout techniques based on AFM include cantilever arrays that
allow for analysis of large areas with high resolution, and can probe for instance mul-
tiple live cells for their elastic properties or the presence of receptors in the cell mem-
brane. Similarly, coating a parallel array of cantilever with a different material or
reagent on each lever results in a “chemical nose” that can sense a variety of chemicals
or toxins in very small volumes or could read out the viscosity of sample fl uid.
6.2
Surface Chemical Array Modifi cations
A novel technique that has been applied more and more in recent years to construct
stable nanoscale patterns on surfaces is dip pen nanolithography (DPN) (Piner et al.
1999
). The technique is based on the localized deposition of reagents from the AFM
tip to the surface using a solvent meniscus between the tip and sample surface. The
main benefi t of DPN is that it is a one-step process, does not require resists, and can
achieve line widths of 10-15 nm, a resolution not achieved with photolithography
or microcontact printing (Quist et al.
2005b
). Bonding of reagent to the sample
surface is usually achieved by chemisorption through thiol chemistry or by electro-
static interactions. Using DPN, it is possible to create high-density arrays of, for
instance, DNA or protein (Fan et al.
2002
) or to write structures with conducting
polymers on charged semiconductor surfaces (Lim and Mirkin
2002
) that can be
used for assay chip technology.