Biomedical Engineering Reference
In-Depth Information
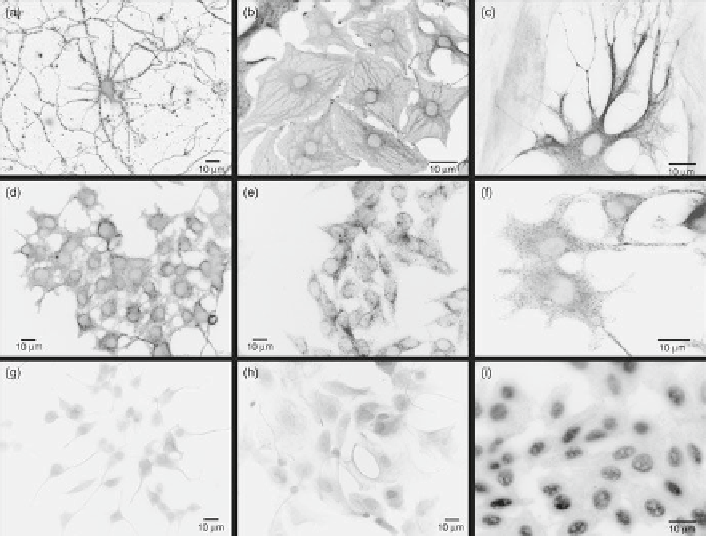
Fig. 2
Fluorescent labeling of neurons and glia with antibody-conjugated 605-nm quantum dots.
(
a
) Primary cortical neurons specifi cally labeled for b-tubulin. (
b
,
c
) Primary cortical astrocytes
specifi cally labeled for GFAP. (
d
,
f
) PC12 cells labeled for b-tubulin. (
e
) r-MC1 neural retinal
Muller glial cells specifi cally labeled for GFAP. (
g
) PC12 cells labeled for b-tubulin using standard
ICC. (
h
) Primary spinal cord astrocytes labeled for GFAP using standard ICC. (
i
) An example of
artifactual nonspecifi c labeling in r-MC1 Muller cells with anti-GFAP-conjugated 605-nm quantum
dots. In this case, putative nonspecifi c electrostatic interactions between quantum dots and cellular
proteins led to intense nuclear staining and mild cytoplasmic staining using other quantum dot con-
jugation protocols described for mammalian cells. All imaging parameters were constant for the
different experimental conditions, with an acquisition/exposure time of 30 ms for all panels
except (
i
), which was taken with an acquisition time of 100 ms. Reproduced from Pathak et al. (
2006
)
4
E f fi cacy of Different Antibody Conjugation Methods
to Quantum Dots
One critical issue that has not been addressed is experimentally determining the
number of antibodies bound to quantum dots which are functionally available for
target protein binding (Pathak et al.
2007
). This is critical for the analysis and proper
interpretation of biological data labeled using these kinds of methods. While other
groups have qualitatively characterized antibody-functionalized quantum dots using
TEM, AFM, UV spectroscopy, and gel electrophoresis, and in some cases have sug-
gested estimates of the putative number of total antibodies bound to quantum, no
calculations of the number of functional antibodies bound to quantum dots based on