Biomedical Engineering Reference
In-Depth Information
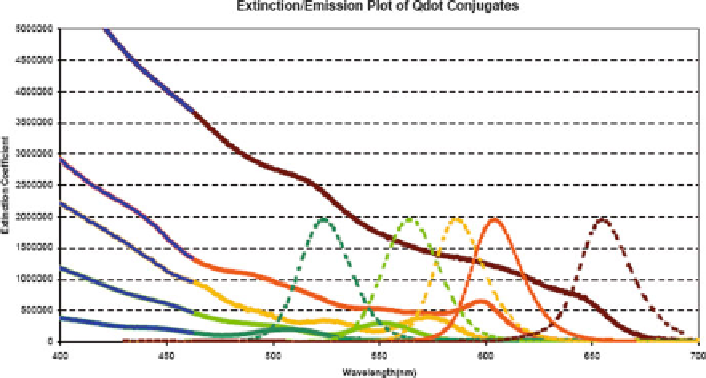
Fig. 1
Characteristic absorbance and emission spectra for a series of fi ve different quantum dot
sizes. Absorbance spectra are characterized by broad band excitation that most effi ciently excites
at lower wavelengths (denoted by the
blue
portion of absorbance curves). Emission spectra are
characterized by symmetric, narrow Gaussian-shaped curves, which shift to higher wavelengths
with increasing quantum dot size.
Source
:
Quantum Dot Corp
,
http://www.qdot.corp
electrons, and holes, and QDs, unlike their bulk semiconductor materials, possess
quantized energy levels (Murray et al.
2000
; Parak et al.
2003
; Michalet et al.
2005
) .
These energy levels increase with decreasing QD size and QDs and by varying the
size and chemical core composition such that QDs' luminescence spans across the
visible spectrum (Nirmal and Brus
1999
). Another consequence of the QD size
being smaller than the Bohr radii for the material is that the semiconductor material
now has a greater probability of absorbing light at higher energies (shorter wave-
length) and emitting light at high energies (shorter wavelength). Figure
1
shows a
typical QD absorption and emission spectrum. Absorption of a photon with energy
above the semiconductor band gap energy, the energy difference between the top of
the valence band and the bottom of the conduction band, results in the creation of an
electron-hole pair that has a broadband absorption spectrum with increased proba-
bility at shorter wavelengths (Michalet et al.
2005
). As the QD size decreases, elec-
tron-hole pairs experience stronger confi nement and produce shorter wavelength
(higher energies) when photoexcited. The radiative recombination of electron-hole
pairs produces an emission of a photon that possesses a narrow and symmetric
energy band. Thus excitation spectra track that of absorbance spectra and will emit
with the same wavelength spectrum independent of the excitation wavelength
(Bruchez et al.
1998
). Taken together, semiconductor QDs possess a unique absor-
bance and emission spectrum as a physical consequence of their nanometer size.
Currently, QDs are produced from a variety of materials such as: cadmium sulfi de,
cadmium selenide, cadmium telluride (periodic groups II-VI), or gallium arsenide
and indium phosphide (III-V), or lead sulfi de, lead selenide, silicon, and germanium