Biomedical Engineering Reference
In-Depth Information
Fig. 11
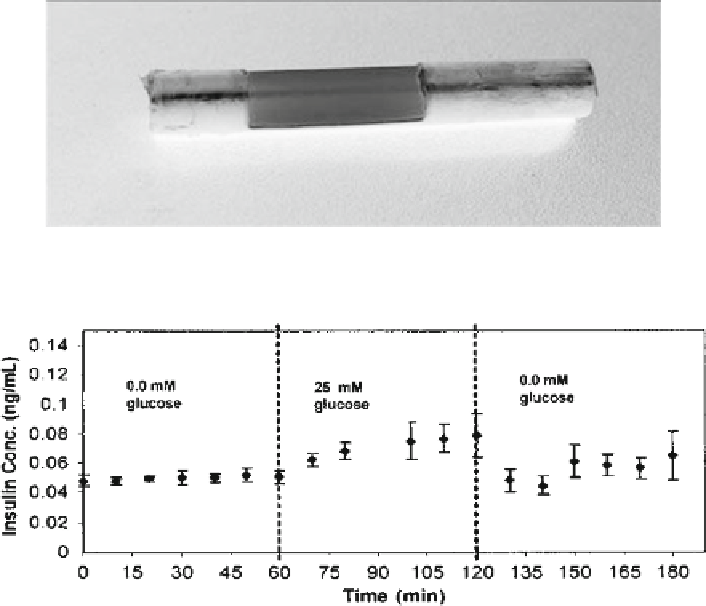
A nanoporous capsule for encapsulating insulin producing cells
Fig. 12
Response of a nanoporous capsule encapsulated insulionma cells to alternating low and
high (
center
) glucose concentrations
larger diameter and thicker membranes than the multistep micromachining techniques
described in Leoni and Desai (
2004
); however, they still have more precise pores
and thinner membranes than polymeric devices (Fig.
11
). Capsules with membranes
containing 75-nm pores effectively passed glucose and hindered the passage of IgG.
The diffusion coeffi cients of glucose and IgG through the membranes were 1.58E-
06 cm
2
/s and 4.09E-10 cm
2
/s, respectively. The diffusion of glucose is comparable
to other encapsulation devices while the diffusion of IgG is signifi cantly lower than
with other encapsulation devices (Burczak et al.
1994
; Leoni and Desai
2001
) . To
determine the functionality of insulin producing cells in the capsule, the capsule
(75 nm pores) was fi lled with insulinoma cells suspended in a collagen gel. The
cells in the capsule were starved of glucose for approximately 24 h to bring insulin
production to basal levels. The capsules were then placed in a perfusion chamber
and the cells were exposed to a step-increase in glucose in the perfusion media.
A resultant release of insulin from the capsules was observed indicating that the
glucose enters the capsule from the outside environment and the cells can respond
to the glucose by releasing insulin, which then diffuses out of the capsule into the
surrounding environment. Changes in applied glucose concentrations were also