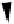Biomedical Engineering Reference
In-Depth Information
O
2
H
2
O
H
2
O
MMO
CH
4
CH
3
OH
HCHO
HCOOH
CO
2
NAD
+
NADH
+ H
+
PQQ
PQQH
2
NAD
+
NAD
+
NADH
+ H
+
NADH
+ H
+
Cells
FIGURE 2.1 Methane oxidation pathway of methanotrophic
microorganisms. Source: Adapted from Chang (1995).
bacterial metabolism of C
1
compounds (Heijthuijsen and Hansen, 1990;
Komagata, 1990). Consequently, a large number of methanol-utilizing
bacteria have been isolated from a wide variety of natural sources, and
most of these isolates have been identified as aerobic, Gram-negative
bacteria (Komagata, 1990).
Methanol is also an intermediate of the methane oxidation pathway
used by methanotrophic organisms (Figure 2.1); methanotrophs are
ubiquitous in nature. Methanotrophs oxidize methane to methanol by
the enzyme methane monooxygenase. Methanol is then oxidized to
formaldehyde by methanol dehydrogenase (Lehninger et al., 1993;
Smeraldi et al., 1994), which is then assimilated into cell material by the
activity of either of two pathways, one involving the formation of the
amino acid serine and the other proceeding through the synthesis of
sugars (Prescott et al., 1996).
A
VAILABILITY OF
E
LECTRON
A
CCEPTORS
Microorganisms obtain energy
by transferring electrons from electron donors (in this case, methanol)
to electron acceptors. Electron acceptors are compounds that have a
lower oxidization state than electron donors; they include molecular
oxygen, nitrate, Fe(III), sulfate, and carbon dioxide. The most energeti-
cally favored mechanism by which microorganisms oxidize organic
compounds is aerobic metabolism (i.e., the use of oxygen as the
electron acceptor) (Table 2.4).
The presence of oxygen is a requirement for obligate aerobic micro-
organisms. Facultative aerobic and anaerobic microorganisms are able
to use other electron acceptors when oxygen is not available. Oxygen is