Biomedical Engineering Reference
In-Depth Information
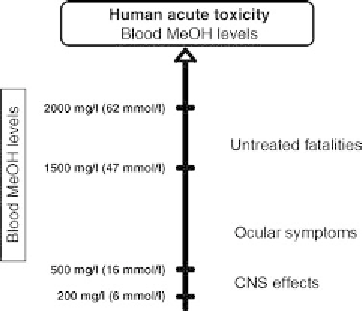
FIGURE 7.12 Blood MeOH levels measured from human cases of acute
toxicity. Blood MeOH levels (mmol/l and equivalent mg/l) with cor-
responding symptoms of toxicity. Source: Modified from WHO (1997),
Environm ental Health Criteria: http:// www.inchem.org /documents /ehc/e hc/
ehc196 .htm#Sec tionN umber: 1.3 Access date: 03/10 /2012.
such as fomepizole, which is a competitive inhibitor of ADH1, are also
common interventions (Hovda and Jacobsen, 2008; Brent, 2009).
MeOH acute toxicity in animal models is completely different.
Although primates, as expected, exhibit similar symptoms (ocular
toxicity, metabolic acidosis) (Baumbach et al., 1977), rodents and
rabbits do not exhibit either of these manifestations (Roe, 1955).
Our laboratory has performed single dose (2 g/kg MeOH), 2-day
and 15-day (daily 2 g/kg doses of MeOH on consecutive days)
pharmacokinetic studies which found no accumulation of MeOH
or FA in mice, whereas a sustained accumulation of both MeOH
and FA was observed in rabbits following a single dose of MeOH
(Sweeting and Wells, 2010; McCallum et al., 2011a,b). However, as
previously reported, no evidence of ocular toxicity or metabolic
acidosis was observed in either mice or rabbits, despite greater FA
accumulation in the latter, and both species appeared normal through-
out the studies.
Although acute MeOH toxicity in humans is thought to be caused by
FA accumulation and the resulting metabolic acidosis, the mechanism