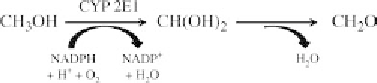Biomedical Engineering Reference
In-Depth Information
FIGURE 7.5 Role of cytochrome P450 2E1 (CYP2E1) in the metabolism of
MeOH. In the presence of NADPH and molecular oxygen, CYP2E1 oxidizes
MeOH into formaldehyde (CH
2
O).
MEOS system principally relies on the cytochromes P450 (CYP)
isozymes CYP2E1 and CYP1A2 for alcohol metabolism and is depen-
dent on the presence of molecular oxygen and NADPH as the cofactor
(Kunitoh et al., 1993; Lieber, 2004) (Figure 7.5).
7.2.1.4 Formaldehyde Dehydrogenase (ADH3) The half-life of form-
aldehyde is approximately 1.5 minutes (McMartin et al., 1979) due to its
rapid oxidation into FA and/or the formation of macromolecular
adducts (Shaham et al., 1996). Using aldehyde dehydrogenase
(ALDH2), formaldehyde can be metabolized into FA in the mitochon-
dria (Teng et al., 2001). Additionally, ADH3 can oxidize formaldehyde
into FA in both humans and rodents through a glutathione (GSH)-
dependent mechanism (Uotila and Koivusalo, 1974; Harris et al., 2004)
(Figure 7.6). ADH3 is approximately 100-fold more efficient at oxi-
dizing formaldehyde than ALDH2, suggesting that it is the predominant
pathway for formaldehyde metabolism in vivo (Teng et al., 2001).
During development, rat and mouse embryos have similar ADH3-
specific activity (Harris et al., 2003).
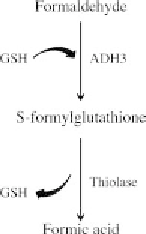
FIGURE 7.6 Role of formaldehyde dehydrogenase (ADH3) in the metabo-
lism of formaldehyde. ADH3 conjugates glutathione (GSH) to formaldehyde
forming S-formylglutathione, which is subsequently metabolized into FA
through the removal of GSH by thiolase.