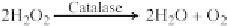Biomedical Engineering Reference
In-Depth Information
TABLE 7.5 Changes in Peroxidative Catalase Activity, Using MeOH As
a Substrate, Throughout Development in Mice and Rats
Peroxidative Activity of
Catalase
Model
Age
Organ
Citation
Mouse
6-12
somites
Whole
embryo
2 nmol formaldehyde
formed/min/mg protein
Harris et al.
(2003)
21-28
somites
Whole
embryo
21 nmol formaldehyde
formed/min/mg protein
Harris et al.
(2003)
Adult
Blood
32.5 U/mg protein
Ishii et al.
(1996)
Rat
6-12
somites
Whole
embryo
7.5 nmol formaldehyde
formed/min/mg protein
Harris et al.
(2003)
21-28
somites
Whole
embryo
17 nmol formaldehyde
formed/min/mg protein
Harris et al.
(2003)
Adult
Blood
55 U/mg hemoglobin
Breinholt
et al. (2000)
activity of catalase, antioxidative catalase activity follows Michaelis-
Menten kinetics only at low concentrations of H
2
O
2
because of the high
reaction rate of this process and the ability of high H
2
O
2
levels to
inactivate the enzyme. Accordingly, only theoretical kinetic parameters
for antioxidative catalase may be calculated, where an apparent K
m
of
25mM has been reported for H
2
O
2
(Vetrano et al., 2005). As such, the
apparent affinity of rodent catalase for H
2
O
2
is approximately 20-fold
lower than that for MeOH (see section titled Peroxidative Role),
indicating that MeOH will be preferentially metabolized over H
2
O
2
.
With less H
2
O
2
being scavenged, the potential for ROS formation is
enhanced, leading to an increased possibility of oxidative damage.
7.2.1.3 Cytochrome P450 (CYP) 2E1 Alcohols including EtOH and
MeOH can be metabolized by a microsomal EtOH oxidizing system
(MEOS) to their respective aldehydes (Teschke et al., 1974). The
FIGURE 7.4 Role of catalase in the metabolism of reactive oxygen species
(ROS): antioxidative activity. H
2
O
2
is scavenged by the antioxidative function of
catalase in all species, forming the nontoxic metabolites of water and oxygen.