Biology Reference
In-Depth Information
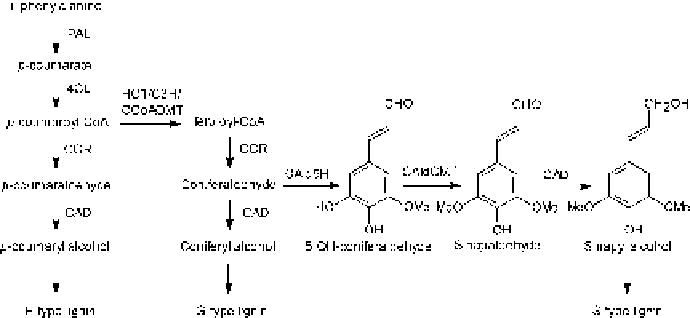
Fig. 8. Biosynthesis of S-lignin in hardwood species. The enzymes coniferaldehyde-
5-hydroxylase (CAld5H) and 5-hydroxyconiferaldehyde O-methyltransferase (CAl-
dOMT) required for biosynthesis of S-lignin are absent in conifers.
S-lignin formation in conifers, especially in wood types that are rich in
H-lignin, such as compression wood.
However, it seems possible that generation of S-lignin in conifers not only
requires overexpression of CAld5H and CAldOMT. Suppression studies in
M. sativa indicated that biosynthesis of S-type lignin might involve methyl-
transferases other than CCoAOMT (
Chen et al., 2006; Nakashima et al.,
2008
). In addition, angiosperm species contain CAD isozymes that efficiently
convert sinapaldehyde to sinapyl alcohol (
Barakate et al., 2011; Sibout et al.,
2003
), and this seems not to apply to conifers (
O'Malley et al., 1992
; Wagner
et al., unpublished results). In addition, peroxidases specific for the one-
electron oxidation of sinapyl alcohol have been identified in angiosperms
(
G
´
mez Ros et al., 2007; Ros Barcel
´
et al., 2007
), but are unlikely to exist in
conifers. Furthermore, transcription factors specific for S-lignin have been
recently identified in angiosperms (
Zhao et al., 2010
).
Another issue that requires investigation is the transport of monolignols
to the apoplast, as it is currently unknown how efficiently monolignols or
monomers that are novel to conifers would get transported to the cell wall.
Lignin design concepts such as the introduction of S-lignin in pine would also
hugely benefit from investigations into the cellular organisation and localisa-
tion of monolignol biosynthesis in conifers.
2. Incorporation of monolignol substitutes into conifer lignins
More recent concepts to facilitate biomass processing include a complete
redesign of the lignin polymer with the intention of creating a biologically
functional polymer that contains chemically labile linkages or linkages