Biology Reference
In-Depth Information
and coniferyl alcohol (
Ros Barcel ´ et al., 2004
). However, this situation is
not so clear in the case of sinapyl alcohol; in this case, typical acidic perox-
idases, with some exceptions (
Christensen et al., 1998
), are generally regarded
as poor catalysts.
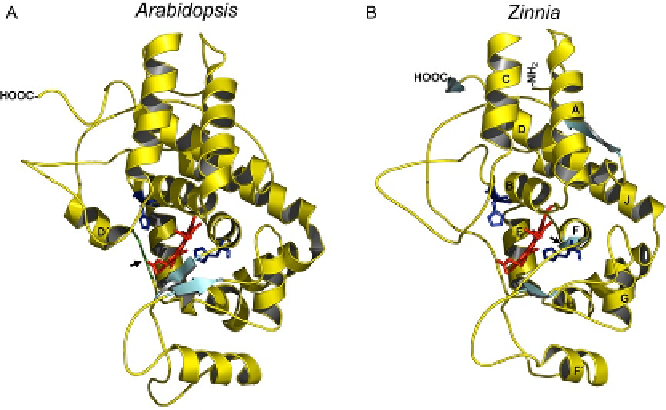
Theoretical comparative models between Zinnia elegans S peroxidase and
Arabidopsis thaliana G peroxidase showed two important differences
(
Fig. 10
). Helix D
0
in Arabidopsis fixes the motif which determines the
conformation and hydrophobicity of the substrate-binding site (
Østergaard
et al., 2000
) and it is absent in S peroxidases (
Ros Barcel ´ et al., 2007
). On the
contrary, there is a novel
b
-strand in S peroxidases that influences the
catalytic centre of the enzymes. All of these factors are likely to condition
the substrate specificity of S peroxidases, determining the unique catalytic
properties (
G
´
mez Ros et al., 2007a; Ros Barcel
´
et al., 2007
).
G
´
mez Ros et al. (2007a)
determined the structural motifs of S peroxi-
dases, by alignment of the amino acid sequence of a set of peroxidases whose
capacity for oxidizing S moieties is well known (
Gabald
´
n et al., 2005;
Quiroga et al., 2000; Sasaki et al., 2006; Takeda et al., 2003
) with two typical
G peroxidases (
Nielsen et al., 2001; Østergaard et al., 2000
).
Searching through protein databases for other S peroxidases in angios-
perms showed that the determinants of S peroxidases have been conserved
Fig. 10. Predicted 3D structure of the Arabidopsis thaliana ATP A2 G peroxidase
(A) and the Zinnia elegans S peroxidase (B). Adapted from
Ros Barcel ´ et al. (2007)
,
reproduced with permission from Elsevier.