Biology Reference
In-Depth Information
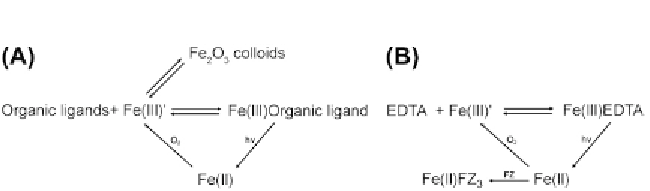
Figure 3.1
Fe speciation chemistry.
Fukushima, 2004
;
Rue & Bruland, 1995
;
Wu & Luther, 1995
). These organic
ligands may be classified according to their conditional stability constants
with respect to Fe(III) - the stronger L1 class and the weaker L2 class (e.g.
Gledhill & van den Berg, 1994
). A common view is that L1 is composed of
siderophore (strong Fe-specific chelators secreted by microorganisms dis-
cussed in further detail in Sections
2.1 and 2.2
)-like compounds, whereas
L2 is made up of cell degradation products. However, a more complex pic-
ture is now emerging with the understanding that many compounds with
Fe-binding abilities are released into the water via active secretions, graz-
ing and cell lysis - contributing to an 'Fe-ligand soup' in aquatic environ-
ments (
Hunter & Boyd, 2007
). Because these compounds are often found in
10
3
-10
5
times higher concentrations than iron, they affect the composition
of the Fe pool significantly, even though their binding constants are not as
high as those of siderophores.
Fe speciation must also be taken into account in laboratory work. Inor-
ganic Fe species (e.g. FeCl
3
) will precipitate out of solution as Fe hydroxides
whose speciation and stability fluctuate over time. Therefore, in order to
work with well-defined Fe substrates as well as with known dissolved iron
concentrations, Fe(III) must be chelated before use (Fig.
3
.
1
B and
Kranzler,
Lis, Shaked, & Keren, 2011
).
1.2. The Iron Hypothesis
Dissolved iron concentrations in many aquatic environments are in the
nanomolar to picomolar range (
Johnson, Gordon, & Coale, 1997
). Large
regions of the ocean termed 'high-nitrate low-chlorophyll' (HNLC)
regions are characterized by sufficient macronutrient concentrations but
low chlorophyll concentrations. These regions are also characterized by
picomolar levels of dissolved Fe (
Martin, Gordon, Fitzwater, & Broenkow,
1989
). This observation led to John Martin's Iron Hypothesis, which sug-
gested that photoautotrophic growth in these large regions of the ocean is
in fact limited by low Fe availability (
Martin et al., 1994
). Numerous iron