Biology Reference
In-Depth Information
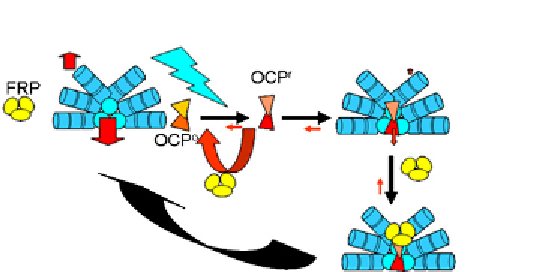
Figure 1.4
Schematic of the current understanding of the OCP photoprotective mecha-
nism. Light (thunderbolt) activates the OCP (fused triangles) converting OCP
o
into OCP
r
.
Only OCP
r
is able to bind to the core of phycobilisomes or to FRP (ovals, here shown as a
trimer). These interactions are light independent. Fluorescence quenching depends on
the concentration of OCP
r
and on the FRP/OCP ratio. Vertical arrows symbolize energy
flow, either to the reaction centre or dissipated as heat. See the colour plate.
are needed for attachment to the phycobilisome and fluorescence quench-
ing (
Gwizdala, Wilson, et al., 2011
;
Wilson, Gwizdala, et al., 2012
;
Wilson,
Punginelli, et al., 2008
). Only the activated red protein is able to bind to
the phycobilisomes (
Gwizdala, Wilson, et al., 2011
;
Wilson, Gwizdala, et al.,
2012
). In the cells, the amplitude of fluorescence quenching depends on the
concentration of the OCP
r
and on the affinity of OCP
r
for the phycobili-
some. The OCP
r
can also interact with FRP, which facilitates rapid reversion
to the orange, inactive form. Thus, fluorescence quenching is affected by the
presence and concentration of the FRP and on its affinity of OCP
r
. How
organisms that lack an FRP accomplish this step is an interesting question
although it is known that the OCP
r
can thermally convert back to OCP
o
without interaction with any protein. Thus, the stability of the unbound
OCP
r
also influences the rate and amplitude of fluorescence quenching
(
Gwizdala, Wilson, et al., 2011
;
Kuzminov, Karapetyan, et al., 2012
;
Wilson,
Gwizdala, et al., 2012
). The attachment of OCP
r
to the phycobilisomes sta-
bilizes the red activated form, presumably remaining attached until interac-
tion with the FRP. The phycobilisome-bound OCP
r
quenches the absorbed
light energy and fluorescence via charge transfer between a bilin of an
APC
660
trimer and the carotenoid of OCP
r
or by EET between the excited
state of the bilin and the S1 state of the hECN in OCP
r
. FRP, by interacting
with the attached OCP
r
, may induce its conversion to OCP
o
and its almost
simultaneous detachment from the phycobilisome. Under strong illumina-
tion, a new OCP
r
will rapidly attach to the phycobilisome that will 'remain'
quenched. In darkness or low light, the concentration of OCP
r
is null or