Biology Reference
In-Depth Information
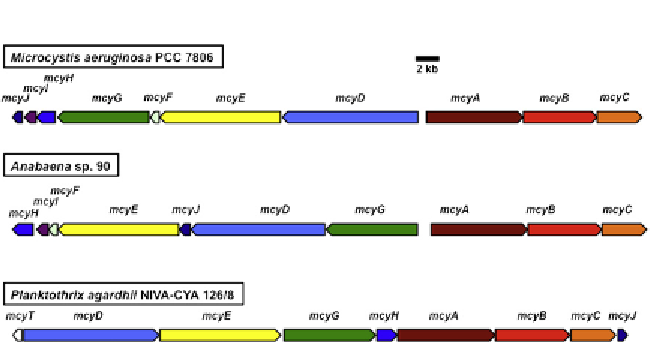
Figure 6.2
Comparisonofthreeselected
mcy
geneclustersfromdiferentgenera.Note
thatthe
Planktothrix agardhii mcy
clusterlacksthe
mcyI
and
mcyF
genes(implicatedin
thebiosynthesisof
erythro
-2-methyl-
d
-Asp)andhasasupplementarytype-IIthioester-
asegene,
mcyT
.Seethecolourplate.
However, the transcription of the
mcy
genes is repressed in the dark and
expressed by light, and thus, the production of microcystins occurs essen-
tially during the light period (
Straub, Quillardet, Vergalli, de Marsac, &
Humbert, 2011
).
A biosynthetic scheme, for microcystins, was proposed based on the bio-
informatic analysis of the
mcy
cluster, involving two PKSs, one hybrid PKS/
NRPS and three NRPSs, one tailoring enzyme and one ABC transporter
(
Tillett et al., 2000
). The biosynthesis probably starts with the loading of
phenyllactate on McyG followed by extension and methylation (
Fig. 6.3
).
In vitro experiments conducted on the isolated adenylation domain and
peptidyl carrier protein domain (A-PCP domains) of McyG showed that
the preferred starter for McyG is probably phenyllactate rather than phen-
ylacetate as first proposed (
Hicks, Moffitt, Beer, Moore, & Kelleher, 2006
).
However, the carboxylate of the phenyllactate starter should be lost to give
the Adda moiety by an unspecified step. McyJ, a tailoring enzyme, is believed
to catalyse the O-methylation on the McyG product as evidenced by genetic
inactivation of
mcyJ
(
Christiansen et al., 2003
). The chain is then loaded on
McyD for extension on two modules, and then on McyE for extension,
amino transfer and condensation of D-glutamate. The D-glutamate is teth-
ered to the PCP by its γ-carboxylate, and thus, the next peptide bond is a
γ-bond rather than an α-bond. A first genetic study (
Nishizawa, Asayama,
& Shirai, 2001
) attributed the glutamate racemase activity to McyF but