Biology Reference
In-Depth Information
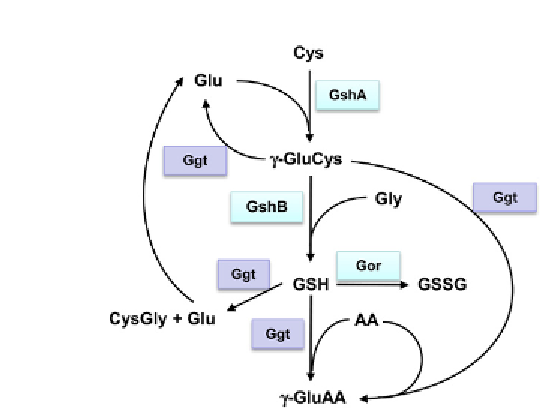
Figure 5.2
The glutahione metabolism.
AA: One amino acid or a dipeptide, Cys:
Cysteine, Ggt: γ-glutamyltranspeptidase, Glu: Glutamate, Gly: Glycine, GR: Glutathione
reductase, GSH: Glutathione (reduced form), GshA: γ-glutamyl-cysteine ligase, GshB:
Glutathione synthase, GSSG: Glutathione disulfide (oxidized glutathione), γ-GluAA:
γ-glutamyl-aminoacid, γ-GluCys: γ-glutamyl-cysteine.
located downstream of a gene encoding an anti-oxidant glutaredoxin (Grx)
enzyme (
Table 5.2
), in accordance with the close relations between GSH
and Grx enzymes (see below section 5). The attempted deletion of the
gshA
gene from the chromosome of the cyanobacterium
Synechocystis
PCC
6803, which is polyploid (
Griese et al., 2011
;
Labarre et al., 1989
), invari-
ably yielded a heteroploid strain harbouring both mutant (Δ
gshA
) and
wild-type (
gshA
+
) copies of the chromosome (
Cameron & Pakrasi, 2010
).
We too failed to delete
gshA
from all
Synechocystis
PCC 6803 chromo-
some copies, even in cells incubated with various amounts of exogenous
GSH, or GSH plus Fe mixtures, to try complementing for the lack of GshA
activity. In contrast, a fully segregated
gshB
-deleted mutant was obtained in
both
Synechococcus
PCC 7942 (
Okumura, Masamoto, & Wada, 1997
) and
Synechocystis
PCC 6803 (
Cameron & Pakrasi, 2010
;
Narainsamy, Cassier-
Chauvat, Junot, & Chauvat, 2011
). The
Synechocystis
PCC 6803
gshB
-less
mutant lacks GSH, but instead it accumulates the γ-glutamyl-cysteine GSH
precursor (
Cameron & Pakrasi, 2010
). This mutant grows slower than the
WT strain under favourable laboratory conditions, and it is more sensi-
tive to oxidative stress triggered by H
2
O
2
, rose bengal, methyl viologen
(
Cameron & Pakrasi, 2010
), light plus glucose (
Narainsamy et al., 2011
),
and to the gentamycin antibiotic (
Cameron & Pakrasi, 2011
). In plants,
and a few cyanobacteria (
Table 5.2
), GSH is polymerized by the enzyme