Geography Reference
In-Depth Information
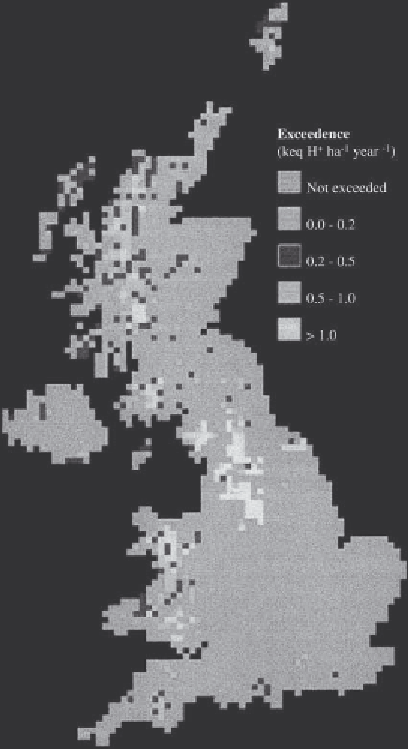
Figure 11.4
Exceedence of critical loads for acidity by
deposition of sulphur and nitrogen to freshwater.
geological weathering, but human activities have
added greatly to these, and it is calculated have
caused global enrichment of selenium, silver,
cadmium, selenium and mercury by 2.8, 3, 7.6,
11.3 and 24.1 times, respectively (Nriagu and
Davidson 1986). Copper smelting at around 7000
BC is one of the earliest instances of human
exploitation of heavy metals (Renfrew and Bahn
1991), but heavy metal extraction and processing
have shown greatest increase since the Industrial
Revolution and particularly during the present
century. Anthropogenic release of heavy metals is
associated with transport, industry, mining,
municipal wastes, agriculture, geothermal
development and waste dump leaching (Foster
and Charlesworth 1997). For some metals, the
dominant source of anthropogenic enrichment
has changed during the last 200 years from mineral
extraction and processing to releases associated
with fossil fuel combustion and product use (Tarr
and Ayres 1990).
Understanding the movement and storage of
heavy metals in fluvial systems is made more
complicated by the fact that a very high
proportion of river transport takes place for many
metals in association with sedimentary particles,
especially the silt and clay fractions.
Concentrations of heavy metals in fine-grained
bed sediments may be five orders of magnitude
greater than concentrations dissolved in the water
column (Horowitz 1991). For some metals, such
as iron, the particulate-associated fraction has been
shown to account for more than 99 per cent of
the total transport in major rivers, such as the
Amazon and Mississippi (Salomons and Förstner
1984). Over the short term, flushing of sediment
and heavy metals in urban drainage during the
early part of storm events may lead to high metal
concentrations as contaminants accumulated on
roofs, in storm drains and especially on road
surfaces (Table 11.4) are removed (e.g. Ellis
et al.
1986; Xanthopolous and Hahn 1993). Over the
longer term, fine sediments with high heavy metal
concentrations may go into floodplain storage
through overbank deposition and channel
accretion, or be deposited in lake, reservoir or
estuarine environments. However, this material
Source:
Critical Loads Advisory Group 1994.
Heavy metals
Metal pollution of freshwaters may pose serious
problems because dissolved heavy metals, such as
arsenic, lead, mercury, cadmium and selenium, are
toxic to humans and animals in low
concentrations. Natural sources of heavy metals
include volcanic and geothermal activity and