Chemistry Reference
In-Depth Information
7
8
2
3
4
6
5
1
0
10
20
Time (min)
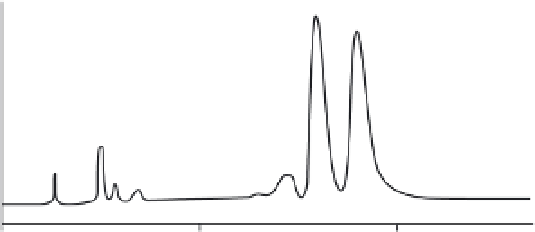
FIGURE 11.6
HPLC separation of carotenoids from an algal source.
OH
H
HO
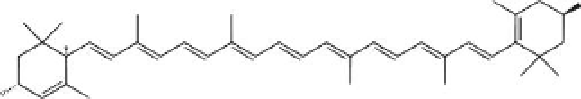
FIGURE 11.7
Chemical structure of lutein.
11. 3 LUTEIN
The name
lutein
comes from the Latin
luteus
, meaning “yellow,” whereas in Chinese
it takes the meaning of “yellow color of leaf.” Lutein is synthesized by plants and
found in reasonable quantities in green leafy vegetables such as spinach and kale.
Lutein is also responsible for the yellow color of egg yolk, chicken skin, and fat.
The long aliphatic carbon chain gives lutein (Figure 11.7) the properties of an anti-
oxidant and as a blue light absorber, thus appearing as a yellow compound—properties
that are important for the protection of the human retina. Lutein has a molecular
formula, C
40
H
56
O
2
, and has a linear aliphatic carbon chain.
It is formed from eight
isoprene units (tetraterpenoid) and two hydroxyl functional groups at the two ends.
11.4 ISOLATION OF LUTEIN
Lutein is generally extracted from plants with organic solvents. As an example, from
marigolds dichloromethane is used. The base material for extraction is often the
saponified oleoresin from the flowers, from which it is possible to obtain a raw crys-
talline product, enriched in the carotenoids. Using solvent mixtures such as hexane
and dichloromethane containing 0.10% N,N-diisoppropylethylamine (DIPEA), crys-
tallization of lutein is achieved at -70°C and crystalline lutein product is obtained
of 98% purity. However, if required it can be further purified by HPLC on a silica-
based nitrile-bonded column as the stationary phase, eluting with a mixture contain-
ing hexane (75%) and dichloromethane (25%) with methanol (0.25%) and DIPEA
(0.1%) as the mobile phase.
Search WWH ::

Custom Search