Chemistry Reference
In-Depth Information
100
80
60
40
20
4000
3000
2000
1600
1200
800
400 cm
-1
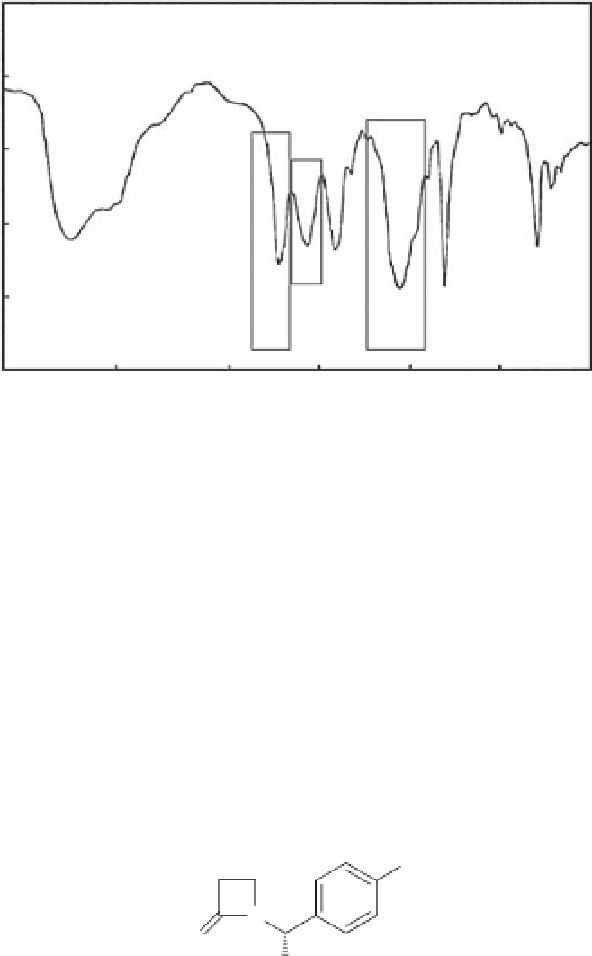
FIGURE 9.9
The IR spectra of monobactams, clearly showing C=O + S=O stretching
bands as shown in the structure (see Figure 9.7).
9.8 NOCARDICINS
Related to the monobactams, the nocardicins also contain a core β-lactam but exhibit
a ring opened attachment with no sulfur in the molecule. The nocardicin antibiotics
were first reported by researchers at Fujisawa Pharmaceutical Co., Japan, in 1976,
from a producing strain of
Nocardia uniformis
subsp.
Tsuyamanensis
ATCC 21806.
These β-lactams are N-acyl derivatives of 3-amino-nocardicinic acid (Figure 9.10).
In total, seven nocardicins were isolated from the metabolites of
Nocardia unifor-
mis
, named nocardicins A-G. Nocardicin A is the major component and also has the
highest activity (Figure 9.11).
H
2
N
OH
N
O
COOH
FIGURE 9.10
The structure of 3-aminonocardicinic acid.
CO
2
H
O
HO
2
C
O
R
O
N
R
NH
2
S
N
OH
Z
H
N
OH
FIGURE 9.11
Structure of nocardicin A.

Search WWH ::

Custom Search