Chemistry Reference
In-Depth Information
S
N
O
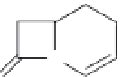
FIGURE 9.5
The backbone structure of cephalosporin is the cephem.
antibiotics are classified into four classes based on their spectrum of activity, resis-
tance to β-lactamase, and their potency differences against gram-positive/negative
organisms. The important difference between penicillin and cephalosporin struc-
tures can be seen in the enlargement from a ive-membered to six-membered ring
attached to the common β-lactam core. The backbone of the cephalosporin is cephem
(Figure 9.5), which consists of a bicycle system with a four-membered β- lactam ring
and a hydrocyclothiazide ring. Compared to the ive-member hydrothiazole ring, the
cephalosporins exhibit less ring strain than penicillin. Hence, the potency is rela-
tively lower than penicillin. However, they are more stable under acidic conditions
and exhibit fewer allergic reactions; these cephalosporins have a prominent place in
antibiotic therapy in modern times.
9.5 ISOLATION OF CEPHALOSPORIN C
The structure of cephalosporin C is shown in Figure 9.6.
Isolation from fermentation
broth is achieved using macroporous, reverse phase, nonionic adsorption resins. One
of the commercial resins is known as the Amberlite XAD-n resins (where n varies
from 2 to 16 depending on crosslinking). The filtered broth is poured through the
XAD resin, which adsorbs the cephalosporins from the solution. The cephalospo-
rin compound is then eluted from the resin with an aqueous solution containing an
anionic surface active agent, followed by lyophilization, precipitation, and crystal-
lization. The advantage of using adsorption resins is the high efficiency although it
adds cost to large-scale production.
9.6 MONOBACTAMS
In a desire to find ever more useful ß-lactam compounds, the pharmaceutical indus-
try in particular devised new screening techniques and new sources of microbial pro-
ducers. One outcome was the discovery of the monobactams. The term
monobactam
described the novel group of monocyclic bacterially produced ß-lactam antibiot-
ics having a simple core structure, characterized by the 2-oxoazetidine-1-sulfonic
O
H
H
N
S
HO
O
N
O
NH
2
O
O
O
OH
FIGURE 9.6
Structure of cephalosporin C.


Search WWH ::

Custom Search