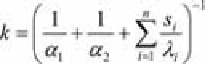Environmental Engineering Reference
In-Depth Information
Table 3.2
Heat capacity
c
for Some Materials at
ϑ
= 0-100°C
Name
c
in
c
in
Name
c
in
c
in
Wh/(kg K) kJ/(kg K)
Wh/(kg K) kJ/(kg K)
Aluminium
0.244
0.879
Copper
0.109
0.394
Ice (-20°C to 0°C)
0.58
2.09
Air (dry, 20°C)
0.280
1.007
Iron
0.128
0.456
Brass
0.107
0.385
Ethanol (20°C)
0.665
2.395
Sand, dry
0.22
0.80
Gypsum
0.31
1.1
Water
1.163
4.187
Glass, glass wool
0.233
0.840
Heat transfer fluid
Wood (spruce)
0.58
2.1
Tyfocor55% (50°C)
0.96
3.45
Q
2
Q
1
•
•
s
1
s
2
s
n
Figure 3.1
Heat Transfer through
n
Layers with the Same Surface Area
A
walls does not change the outside ambient temperature, and this is true
whether the ambient temperature is higher or lower than the building
temperature. The
coefficient of heat transfer
(3.6)
can be calculated with the surface coefficient of heat transfer
α
1
and
α
2
of both
sides, the thermal conductivity
λ
i
and the layer thickness
s
i
of all n layers. Table
3.3 shows the heat conductivity
λ
of various materials.
S
OLAR
T
HERMAL
S
YSTEMS FOR
W
ATER
H
EATING
Solar thermal swimming pool heating
This chapter first deals with swimming pool heating. This is not because heated
swimming pools have any ecological advantages - they always draw a high
demand on drinking-quality water and energy. However, the low-temperature
heat demand for pool heating allows the use of simple and economic solar
energy systems, which have seen widespread deployment in this sector.