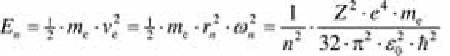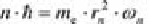Environmental Engineering Reference
In-Depth Information
(4.8)
With this expression, the balance of forces
F
Z
=
F
C
can be resolved for the
orbital radius, which becomes:
(4.9)
and the
angular frequency
:
(4.10)
The integer index
n
describes the orbit number. An electron in orbit
n
contains
the energy:
(4.11)
For instance, the energy of an electron in a hydrogen atom with the proton
number
Z
= 1 at the first orbit (
n
= 1) is
E
1,Z=1
= 13.59 eV.
Elevating an electron from one orbit to the next highest orbit requires the
energy
E
=
E
n
-
E
n
+1
. This energy must be provided from outside the atom.
All orbits can only hold a limited number of electrons. At the first orbit (
n
= 1)
the maximum number is 2 electrons, at the second 8, then 18, 32, 50 and so
on. The electron energy decreases with rising orbit index
n
. For
n
=
∆
it
becomes
E
= 0.
The photo effect
Light, with its photon energy, can provide the energy to lift an electron to a
higher orbit. The
photon energy
is given by:
(4.12)
with the wavelength
and the speed of light c = 2.99792458
•
10
8
m/s. When
a photon with an energy of 13.59 eV hits a hydrogen atom electron in the first
orbit, this energy is sufficient to lift the electron to orbit
E
λ
. In other words, it
totally separates the electron from the nucleus. This energy is also called the
ionization energy. The total release of an electron from the nucleus by photons
is called the
external photoelectric effect
. The photon in the hydrogen example
must have a wavelength lower than
λ
= 90 nm, which places it in the realm of
X-rays.
Because photovoltaic cells mainly convert to electricity photons of visible,
ultraviolet and infrared light, i.e. photons of lower energy than X-rays, the
external photo effect is not applicable to photovoltaic cells. The so-called