Biomedical Engineering Reference
In-Depth Information
PEG chains, which are used for PEGylation of proteins, are not subjected to metabo-
lism, and the elimination mechanism is either liver uptake or degradation by immune
system, depending upon their molecular mass
[388]
.
PEGylation of proteins is usually achieved by a chemical reaction between the
protein and suitably activated PEGylation reagents. Various chemical groups on
the amino acid side chains are being explored for their reaction with the PEG moi-
ety, such as -NH
2
, -NH-, -COOH, -OH, -SH groups as well as disulfide (-S-S-)
bonds. Reactive electrophilic intermediate is often created in PEGylation. Examples
of PEG-protein conjugation reaction are given in Figs. 11.15-11.17.
Table 11.13
illustrates various derivatives of PEG used for PEGylation of P/P drugs. Huang et al.
[389]
used different methods to prepare an adduct of a PEG-lysine copolymer with
either cysteamine or 1-amino-2-methyl-2-propanethiol. Cysteine-containing peptides
were then conjugated to thiol groups on the polymer. The peptide was released intra-
cellularly as the disulfide bond is cleaved by glutathione, a physiologically ubiqui-
tous reducing agent
[389]
.
PEG conjugation arises from the use of either stable or hydrolysable linkages,
the latter resulting in the generation of prodrugs. The resulting conjugated product
shows increased solubility; increased steric hindrance that results in the reduced bind-
ing affinity of P/P, which prolongs the circulation time; increased
in vitro
and
in vivo
biological activity; increased absorption rate and consequently bioavailability; and
altered biodistribution (by limiting diffusion across membranes, it often retains drugs
in the plasma compartment, resulting in a reduced volume of distribution); reduced
rates of kidney clearance and proteolysis together with reduced immunogenicity and
toxicity; increased stability over a wide range of temperature and pH; and high mobil-
ity in solution
[390]
. Thus, PEG-protein conjugation creates an overall improved
physicochemical and pharmacological profile, which is highly influenced by PEG
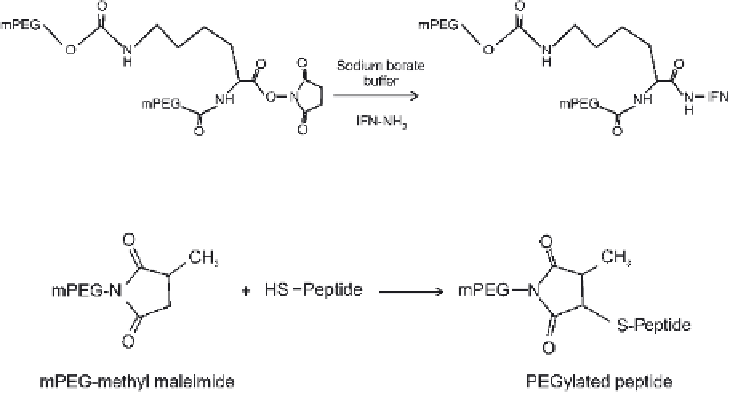
Figure 11.15
PEGylation of INF-2a by branched mPEG
2
-COOH.
Figure 11.16
mPEG methyl maleimide-conjugated peptide.
Search WWH ::

Custom Search