Biomedical Engineering Reference
In-Depth Information
rate, which is quantified in terms of Svedberg (s) units. The prokaryotic RNA is 0s,
whereas generally the eukaryotic RNA is 0s.
An initiation complex is formed by the assembly of one of the ribosomal sub-
units, mRNA, methionine-charged tRNA, and some associated proteins. The initia-
tion complex moves along the mRNA to identify the start codon. Helicase activity
of the associated proteins aids in the unwinding of the mRNA. The protein synthesis
always starts at the AUG, the start codon for methionine. This ensures that mRNA
is read in the correct reading frame. The tRNA that charges the first methionine is
unique in both prokaryotes and eukaryotes and differs from the tRNA that brings
methionine at positions other than the starting one in the growing polypeptide chain.
Hence, only initiator methionine tRNA (tRNA
i
met
) is capable of binding at the P site
on the ribosome, whereas others bind at the A site. As tRNA
i
met
recognizes the start
codon, the movement of the complex is halted, and the larger subunit of the ribosome
also links to form the 0s ribosome.
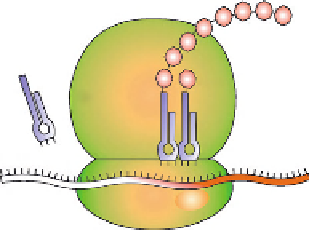
Several proteins called elongation factors now come into play to carry out the
process of polypeptide chain elongation. As discussed earlier, the tRNA
i
met
is at
the P site, which is the site of polypeptide elongation. The next aminoacyl tRNA
is brought at the acceptor (A) site, with the appropriate base pairing between the
codon on mRNA and anticodon on tRNA. GTP hydrolysis and some conforma-
tional changes in the ribosome cause tight binding of charged tRNA at the A site
and bring the amino acid close to the tRNA at the P site. The amino group of
the incoming amino acid reacts with the carboxylic group of the charged methio-
nine to form a peptide bond. This peptidyl transferase activity is catalyzed by larger
rRNA. Subsequent to bond formation, ribosome moves along or translocates by one
codon. After this translocation, the tRNA
i
met
without its methionine is positioned at
the exit (E) site of ribosome and the second tRNA with a dipeptide attached is now
at the P site, leaving the A site empty for the incoming aminoacyl tRNA. The peptide
chain grows in similar fashion (
Fig. 1.
) until it comes across one of the stop codons.
Thereafter, a battery of protein factors bring about the release of the completed poly-
peptide chain. The polypeptide finally assumes its native three-dimensional confor-
mation on release
[-50]
.
Figure 1.7
Translation of mRNA.
Polypeptide
Amino acid
Ribosome
tRNA
mRNA
Search WWH ::

Custom Search