Agriculture Reference
In-Depth Information
um hexametaphosphate adds sodium to the water to make the sodium concentration higher
than the calcium and magnesium; this makes it a better solvent. Less expensive softeners
such as washing soda and trisodium phosphate are very alkaline, which is undesirable for
use on hair and skin. In addition, they precipitate minerals from the water, resulting in a
sludge or film in the wash or rinse water. Sodium hexametaphosphate, by contrast, holds
the minerals in suspension in the water so they cannot form scum. It is tasteless and non-
toxic and has a neutral pH.
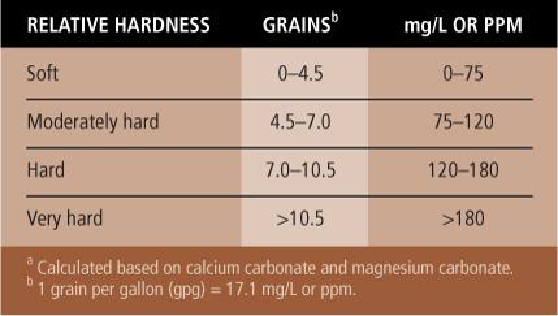
Water hardness
a
This water softener is useful in two ways: to increase the effectiveness of soap and to
act as a thorough rinse. With cold, warm, or hot water, sodium hexametaphosphate helps
soap or shampoo do a better job of cleaning, as it prevents the dingy, insoluble scum from
forming. As a rinse for a horse's hair or a blanket, a sodium hexametaphosphate solution
removes the graying dullness left by previously deposited soap residues. It has a superi-
or ability to combine with and sequester oily and greasy substances, which prevents them
from reacting with the horse's skin or becoming trapped in the fibers of a blanket.
Here are some especially handy uses for a sodium hexametaphosphate solution: to
sponge away the outline of bridle and saddle from a horse that has just finished working;
to rinse the mane or tail without shampooing; to dampen a stable rubber for use as a dust
magnet in the final stages of grooming.
How much sodium hexametaphosphate to use depends on the hardness of the water. One
teaspoon per gallon of water would be adequate for naturally soft water with a hardness
of 4.5 grains or less per gallon. Two tablespoons per gallon would be more appropriate for