Chemistry Reference
In-Depth Information
electrocatalytic reaction and the reaction
becomes an indirect electrolysis [13].
Figure 2.9
Principle of a redox mediatory
reaction. This is for an oxidation process
The features of a mediated electrocatalytic
reaction are as follows:
A catalytic amount of mediator is enough to
carry out indirect electrolysis.
The substrate undergoes a redox conversion
at a potential lower than that required to
effective its direct electrolysis.
Passivation of the electrode as a result of the
formation of non-conductive polymers
during direct electrolysis can be avoided.
Highly selective and efficient redox
conversion can be carried out.
Frequently used mediators for indirect
electrolyses are multivalent metal ions, halide
ions, polycyclic aromatic hydrocarbons such as
naphthalene and anthracene, triarylamines and
nitroxyl radicals. In addition, many mediators
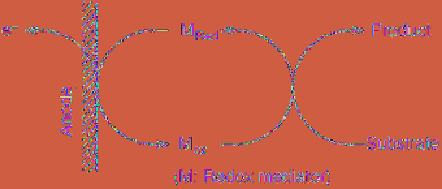
Search WWH ::

Custom Search