Chemistry Reference
In-Depth Information
useful chemicals has been attempted through
either hydrolysis followed by electrolysis or
indirect
electrolysis
with
appropriate
mediators.
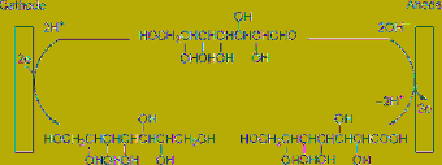
Since water-soluble glucose is available by
hydrolysis of cellulose, its transformation to
gluconic acid and sorbitol has been achieved
with 90% and 50% current efficiency,
respectively, by electrochemical oxidation and
reduction of glucose using an undivided cell, as
shown in
Figure 7.8
. Each of these
electrochemical processes has been practiced
separately on a commercial scale.
Figure 7.8
Paired electrosynthesis of gluconic
acid and sorbitol from glucose
Baizer and his co-workers realized the paired
electrosynthesis of gluconic acid and sorbitol
from glucose using both electrode reactions
[11].
Synthesis of dialdehyde starch was achieved by
oxidation of starch with IO
3
−
mediator using
the Ex-cell indirect electrolysis method (100%
current
efficiency).
Other
various
Search WWH ::

Custom Search