Chemistry Reference
In-Depth Information
hydrogen-overpotential cathodes and
oxygen-overpotential anodes therefore have to
be used for electrochemical reactions in
aqueous acidic and alkaline solutions,
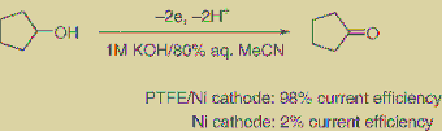
respectively. However, nickel/PTFE
composite-plated Ni electrodes are used for the
reduction and oxidation, as mentioned above,
to provide the corresponding alcohols and
carbonyl compounds, respectively, with much
higher current efficiency compared to unplated
Ni electrodes [96,97]. This is not because of the
higher hydrogen- and oxygen-overpotentials of
the composite-plated electrode suppressing
hydrogen and oxygen evolution but because of
the substrate-collecting effect as a result of
strong hydrophobic interaction between the
hydrophobic electrode surface and hydrophobic
organic substrates [96,97].
(6.29)
6.12.1.2 PTFE-Fibre-Coated Electrodes
PTFE-fibre-coated electrodes, which are
prepared by wrapping the electrode in PTFE
strings, are hydrophobic. It has been
demonstrated that anodic oxidation of
hydroquinones at this electrode in the presence
Search WWH ::

Custom Search