Chemistry Reference
In-Depth Information
Ionic liquids are electrochemically stable and
their usable potential region, i.e. their potential
window where the ionic liquid itself can be
neither oxidized nor reduced, is very large. This
is one of the characteristics of ionic liquids. In
general, oxidative stability depends on the
anion while reductive stability depends on the
cation. However, although the imidazolium ion
has a positive charge, it is more easily oxidized
compared to BF
4
−
owing to the unsaturated
bonds of the imidazole ring. Accordingly, the
oxidative stability of imidazolium ionic liquids
is not always attributable to the oxidative
decomposition of anions. Among organic
anions, trifluoroacetate ion is the most easily
decomposed oxidatively, while (CF
3
SO
2
)
2
N
−
and (CF
3
SO
2
)
3
C
−
have oxidation resistivity and
are not easily decomposed oxidatively. The
order of oxidation resistivity is as follows, and
this order agrees with that of oxidation
potentials measured in a solution:
On the other hand, reductive stability generally
depends on the reductive decomposition of the
cation and the aliphatic ammonium ion is more
difficult to reduce compared to the imidazolium
ion. 2-Methylimidazolium ion is more difficult
to reduce by 0.3-0.5 V compared a
non-substituted imidazolium ion. As shown in
Figure 6.21
, ionic liquids consisting of an
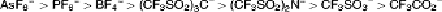
Search WWH ::

Custom Search