Chemistry Reference
In-Depth Information
new anions have been continuously developed
(
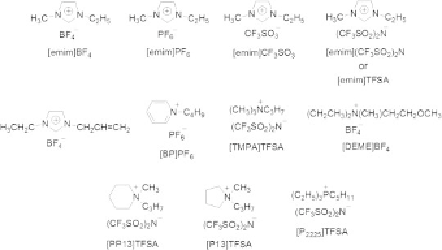
Figure 6.17
).
Figure 6.17
Typical examples of the structures
of ionic liquids and their abbreviations
6.8.2 Hydrophilicity and Hydrophobicity
of Ionic Liquids
Ionic liquids can also be classified as
hydrophilic or hydrophobic, as shown in
Table
6.1
. Regardless of cation type, ionic liquids
having BF
4
−
and CF
3
SO
3
−
as an anion are
hydrophilic while those with PF
6
−
and
(CF
3
SO
2
)
2
N
−
are hydrophobic. As a
characteristic property, hydrophobic ionic
liquids are miscible with neither water nor
ordinary organic solvents like ether and hexane,
and hence they make phase separation resulting
in formation of three phases. The products can
therefore be separated by liquid-liquid
Search WWH ::

Custom Search