Chemistry Reference
In-Depth Information
the electrochemical setup makes it difficult to
obtain high molecular weight polymers.
Recent progress in controlled radical
polymerization using transition metal catalysts
is remarkable both in academia and industry
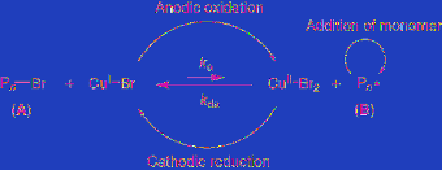
[101]. As shown in
Eq. 5.40
, the equilibrium
(
k
a
/
k
da
) between domant species (A) and active
species (B) controlled by redox of a transition
metal catalyst can control the rate of monomer
consumption, resulting in polymerization with
a controlled manner. The external stimuli by
electrochemical oxidation and reduction of the
metal catalyst change the equilibrium and
consequently more precise control of
polymerization is possible (
Eq. 5.40
) [102].
(5.40)
References
1.
(a) Hammerich, O. and Speiser, B. (eds)
(2014)
Organic Electrochemistry
, 5th edn,
CRC/Taylor & Francis. (b) Torii, S. (2006)
Electroorganic Reduction Synthesis
, Vols 1 and
2, Kodansha and Wiley-VCH Weinheim GmbH.
Search WWH ::

Custom Search