Chemistry Reference
In-Depth Information
(5.11)
Treatment of alcohols with sodium metal
generates hydrogen gas and sodium alkoxide is
formed. This can be explained as follows. The
peripheral electron of sodium metal transfers to
the alcohol to generate a hydrogen radical,
forming hydrogen gas, and the alcohol is
transformed to the alkoxide, in other words
sodium metal acts as a reducing reagent for the
alcohol. When the alcohol is reduced at a
cathode with a low hydrogen overpotential, like
platinum, hydrogen gas evolves and the
corresponding alkoxide is readily formed
without any reducing reagent. It can therefore
be considered that the cathode would act as
sodium metal. This is quite a safe method for
the preparation of alkoxide because harmful
sodium metal is not required. Moreover, the
cathodic reduction of carboxylic acid, phenol,
thiophenol and alcohol is performed in the
presence of quaternary ammonium salt as a
supporting electrolyte to generate the
corresponding highly reactive anions with
quaternary ammonium cation (Q
+
). By using
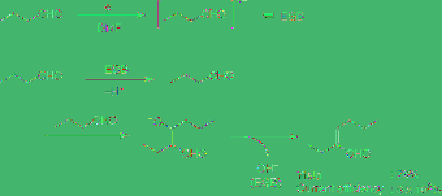
Search WWH ::

Custom Search