Chemistry Reference
In-Depth Information
Figure 5.7
Reactive anion derived from
electrogenerated base (Q
+
= Et
4
N
+
)
In particular, the EGB derived from
2-pyrrolidone is a versatile base and applicable
to organic synthesis such as Stevens
rearrangement, selective α-monoalkylation of
α-(aryl)acetate esters and C-monoalkylation of
1,3-diketones [20,21]. This EGB has also been
demonstrated to be a highly efficient base for
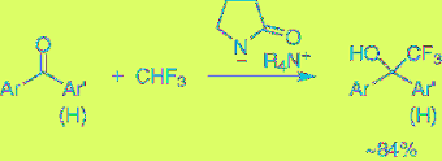
the synthesis of organofluorine compounds. For
instance, it is known that trifluoromethyl anion
is so unstable that it undergoes α-elimination of
fluoride anion to generate fluorocarbene, but
stable trifluoromethyl anion can be generated
by the treatment of fluoroform with this EGB,
and consequently trifluoromethylation of
aromatic aldehydes and ketones is realized to
give the trifluoromethylated alcohols, as shown
in
Eq. 5.7
[22].
(5.7)
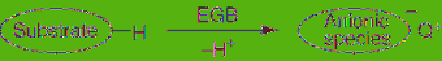
Search WWH ::

Custom Search