Chemistry Reference
In-Depth Information
supporting electrolyte is electrolysed in a
divided cell, the anolyte becomes acidic while
the catholyte becomes alkaline. This is because
hydroxide ions are consumed at the anode
while protons are consumed at the cathode. In a
similar manner, some acid is generated in the
anolyte while some base is generated in the
catholyte during electrolysis in an organic
solvent. Even in an undivided cell, the vicinity
of an anode becomes acidic while that of a
cathode becomes basic during electrolysis.
Anionic species generated cathodically act not
only as nucleophiles but also as bases, and have
interesting reactivities in organic synthesis. The
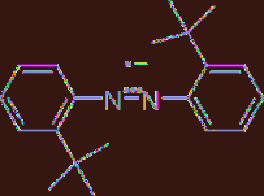
inventor of the cathodic hydrodimerization
process of acrylonitrile, Baizer, demonstrated
that the cathodically generated anion radical of
hindered azobenzene (
Figure 5.6
) could be a
useful base for various organic synthesis, and
he named such bases electrogenerated bases
(EGBs) [19].
Figure 5.6
Electrogenerated base of hindered
azobenzene
Search WWH ::

Custom Search