Chemistry Reference
In-Depth Information
cyclization, respectively, to form large cyclic
lactones by their cathodic reduction using
hydrophobic vitamin B
12
mediator under UV
irradiation [14].
Homo-coupling products are obtained from
aromatic halides using Pd(0) complex as well as
Ni(0) complex as a mediator [15]. The yields
and turnover of the Pd(0) complex are generally
superior to those using Ni(0) complex. The
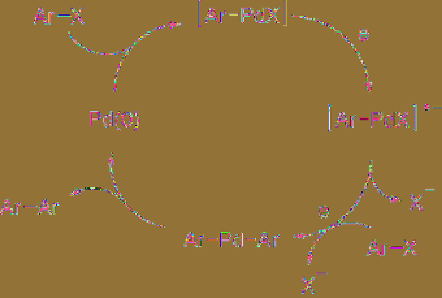
mechanism proposed is shown in
Figure 5.5
.
Aromatic halide reacts with Pd(0) complex to
generate an aryl Pd intermediate, which is
reduced cathodically followed by reaction with
one more aryl halide molecule to form diaryl Pd
complex, resulting in reductive elimination to
give a homo-coupling product. When this
reaction is performed in the presence of CO
2
,
aromatic carboxylic acids are obtained in high
yields [16].
Search WWH ::

Custom Search