Chemistry Reference
In-Depth Information
(4.102)
Reduction of Alcohol and Carboxylic
Acid:
One-electron reduction of these
compounds generates the corresponding
anions, eliminating a hydrogen molecule.
(4.103)
Reduction of Dioxygen:
One-electron
reduction of dioxygen generates superoxide ion,
which is called active oxygen and works as both
oxidant and reductant as well as base and
radical (see section 5.2).
(4.104)
4.7.2.3 Calcogeno (Sulfur, Selenium,
Tellurium) Species
Carcogen compounds are easily oxidized and
oxidation of the corresponding anion generates
radicals that are further oxidized to cations, as
shown in
Eq. 4.105
. These oxidation processes
are usually reversible electron transfers
although the reversibility depends on the
electrolytic conditions.
(4.105)
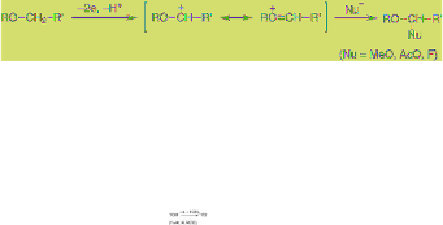
In the case of sulfides, the anodically generated
radical cation at the sulfur atom is attacked by

Search WWH ::

Custom Search