Chemistry Reference
In-Depth Information
potentials of carbanions are extremely low and
their one-electron oxidation generates radicals.
(4.82)
(4.83)
Oxidation of Alkyl Halides:
Alkyl iodides
are more easily reduced and oxidized compared
to the corresponding bromides. Their
one-electron oxidation generates a radical
cation intermediate followed by elimination of
the halogen molecule to generate the alkyl
cation.
(4.84)
Oxidation of Olefins, Ketones, Imines,
and Strain and Cage Compounds:
The
oxidation potential of ketone is generally high,
but even aliphatic ketone is oxidizable.
Although alkane is difficult to oxidize, strain
cyclopropanes and cage-type adamantanes are
relatively easy to oxidize. One-electron
oxidation of those compounds generates radical
cations.
(4.85)

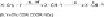
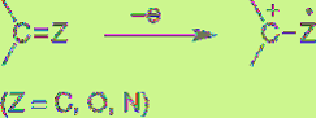
Search WWH ::

Custom Search