Chemistry Reference
In-Depth Information
having a
N
-fluoroalkyl (Rf) group and an
N
-alkyl group, as shown in
Figure 4.4
,
methoxylation takes place at the adjacent
position to the Rf group preferentially. The
regioselectivity increases with an increase in the
electron-withdrawing ability of the Rf group,
i.e. the selectivity increases in the following
order: CH
3
< CH
2
F < CHF
2
< CF
3
[9]. The
mechanism for this regioselectivity outcome is
called kinetic control.
Figure 4.4
Mechanism of regioselective anodic
methoxylation (kinetic control)
The regioselectivity of electrolytic fluorination
is also controlled by kinetic acidity. For
example, in the case of electrochemical
fluorination of heterocyclic compounds, as
shown in
Eq. 4.10
, fluorination proceeds
predominantly via the unstable cation
intermediate adjacent to the carbonyl group
rather than via the stable benzylic cation. This
can be explained in terms of enhanced facile
deprotonation of the anodically generated
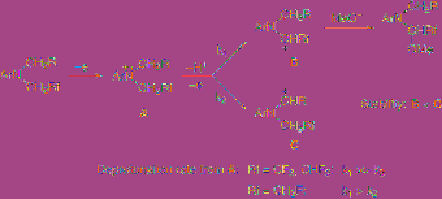
Search WWH ::

Custom Search