Chemistry Reference
In-Depth Information
thermodynamically controlled. Since the
anodically generated radical cation
intermediate seems to be adsorbed on the
anode, deprotonation takes place preferentially
from a less hindered methyl group.
Consequently, this reaction is kinetically
controlled.
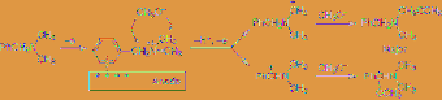
Figure
4.3
Regioselective
anodic
methoxylation
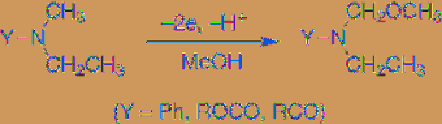
In a similar manner, anodic methoxylation of
N
-ethyl-
N
-methylaniline, the corresponding
carbamate, and amide derivatives also takes
place at the methyl group selectively (
Eq. 4.9
)
[9].
(4.9)
However, the regioselectivity can also be
explained by the difference in deprotonation
rates of radical cation intermediates (so-called
kinetic acidity [10]). For example, in the case of
anodic methoxylation of an aniline derivative
Search WWH ::

Custom Search