Chemistry Reference
In-Depth Information
the phenylimino group can be predominantly
reduced by constant potential electrolysis. This
is because the phenylimino group is more easily
reduced than the alkylimino group as a result of
the electron-withdrawing phenyl group.
However, such selective reduction cannot be
achieved by ordinary reducing reagents.
(4.6)
Similarly, in the case of the molecule with three
halogen atoms shown in
Eq. 4.7
, the halogen
atom at the α-position to the carbonyl group is
most easily reducible, thereby this halogen can
be predominantly reduced at constant potential
electrolysis.
(4.7)
4.3.2.2 Reaction Pathway Selectivity
It is known that the reaction pathway is greatly
changed depending on applied potentials. As
shown in
Eq. 4.8
, one-electron and
two-electron reduction products are obtained

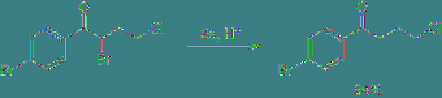
Search WWH ::

Custom Search