Chemistry Reference
In-Depth Information
may be reversed, i.e.
I
′
ad
undergoes subsequent
reaction and then desorption of the resulting
product occurs. Thus, the electrode reaction is
typical in a heterogeneous system, and mass
transfer steps (a) and (g) as well as adsorption
and desorption steps (c) and (e) are involved,
which is quite different from homogeneous
reactions.
Figure 4.2
Elementary processes of electrode
reactions
If the electrode process (electron transfer
process) is abbreviated to E and the chemical
process is abbreviated to C, the organic
electrode reaction can be shown using these
abbreviations. For example, the electrochemical
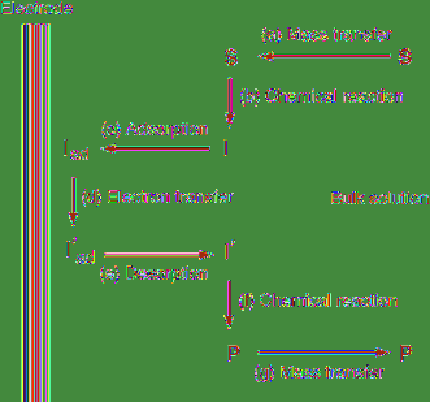
Search WWH ::

Custom Search