Biology Reference
In-Depth Information
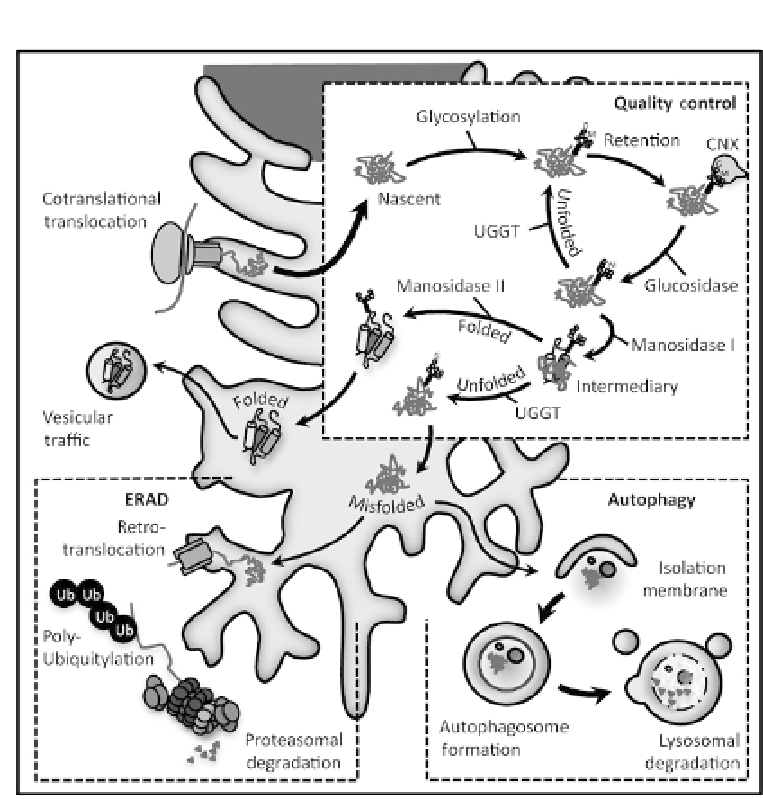
Figure 5.3
Proteins synthesized in the ER are subject to a quality control system
. The lec-
tins, calnexin and calreticulin retain glycosylated proteins in the ER through re/degluco-
sylation cycles until they reach their native conformation. Folded proteins are exported
through vesicle trafficking, while terminally misfolded proteins are degraded through
retrotranslocation and the ubiquitin-proteasome system or autophagy.
and aid in protein folding by inhibiting protein-protein aggregation. In
addition, calnexin and calreticulin trap partially folded or unfolded pro-
teins in the ER (
Hebert et al., 2005
). While correctly folded proteins are
deglucosylated by glucosidase II, then released from calnexin and calre-
ticulin and exported to the Golgi apparatus, misfolded proteins are reglu-
cosylated by the glycoprotein glucosyltransferase and retained in the ER
(
Hebert and Molinari, 2007
). Folding attempts continue with each cycle of
re/deglucosylation up until demannosylation occurs via α-mannosidase I.
Once demannosylated, glycoproteins become a weaker substrate for gluco-
sidase II and glucosyltransferase, thus preventing them from entering new
Search WWH ::

Custom Search