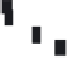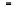Geology Reference
In-Depth Information
0
50
14
C - Uranium series calibration
14
C - Tree-ring calibration
14
C - Cariaco Basin comparison
Uncertainty on sediment ages
Comparison of
14
C and U/Th Clocks
-20
40
-40
-60
30
14
C
U/Th
-80
20
-100
Calibrating the
14
C Clock
-120
10
10
20
30
40
50
-140
Calendar age (x10
3
yr BP)
6
9
12
15
18
21
Age (kybp)
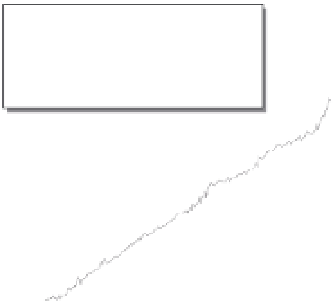
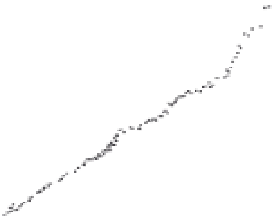
Fig. 3.14
Calibration of
14
C with ocean sediment cores.
High-precision
14
C age of sediment versus age model
derived from correlation of climate proxies from Cariaco
Basin sediment and GISP2 ice core, Greenland. Tree-ring
and U-Th calibration points are also shown, and agree
with the sediment core-deduced offset history. Maximum
offset of roughly 5000 years at about 40 ka is significant
throughout the last glacial interval and declines to the 1 : 1
line in the Holocene. Modified after Hughen
et al.
(2004).
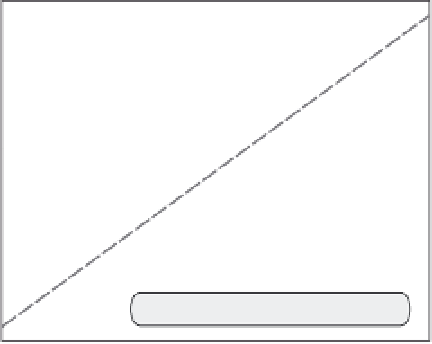
Fig. 3.13
Paired U-Th and radiocarbon ages of corals.
The sampled corals from Barbados grew during rise
of sea level from its Last Glacial Maximum of
roughly −150 m to within 10 m of modern levels. Dates
obtained using both radiocarbon and U-Th series
methods are shown. Two strong spikes in sea-level rise
rates deduced from high slopes on the plot (glacial
meltwater pulses) correspond only if the radiocarbon
ages are systematically shifted to older ages. The U-Th
clock is more trustworthy given its independence from
fluctuations in the rate of cosmic-ray bombardment.
Modified after Bard
et al
. (1990).
the shells of birds. In addition, the gas chro-
matographic methods used for measurement are
relatively inexpensive, making the analysis of
large numbers of samples possible. Only about
2 mg of sample are needed.
Living organisms utilize amino acids only in a
left-handed (or L, for levo-) configuration of their
isomers. Upon the death of an organism, these
restrictive biological processes are terminated,
which frees the amino acids to racemize (flip) into
their right-handed (D, for dextro-) isomeric state.
The ratio of D/L configurations is, therefore, a
clock. The reaction behaves as a first-order revers-
ible chemical reaction in which an equilibrium is
established when the backward reaction (D to L)
balances the forward (L to D). As in all other
chemical reactions, the rate constants are
temperature-dependent, reflecting the Arrhenius
relation. Like the radioactive and cosmogenic
dating methods that entail both production and
decay, this method is commonly restricted to the
period of time over which the measurable ratio
(here the D/L ratio) is changing significantly. The
time to equilibrium varies from one amino acid to
another, and depends strongly upon the thermal
Earth's magnetic field, which modulates the pro-
duction rate of
14
C in the atmosphere.
Amino acid racemization
All fossils contain at least trace amounts of
organic matter than can be retained for long
periods of time. The proteins that constitute this
organic material in both skeletal and shell mate-
rial are themselves composed of large numbers
of amino acids. After death, the amino acids in
these proteins are altered by a set of chemical
and physical processes. The degree to which
this alteration has taken place can be used as a
clock (Bada
et al.
, 1970). Although transforma-
tions represent a very complex set of processes,
some transformations appear to be reliable
enough to provide both a relative and an abso-
lute dating method (Kaufman
et al.
, 1992). Many
types of organic materials have been used, includ-
ing bivalves, gastropods, foraminifera, coral, and