Geology Reference
In-Depth Information
than about 30-40 ka employs carbon-14 (
14
C),
known also as radiocarbon because it radioac-
tively decays. Both
14
C and
13
C are formed in the
atmosphere by cosmic radiation interactions with
atmospheric nitrogen (N), resulting in a mixture
of atmospheric carbon that is roughly 98.9%
12
C,
1.1%
13
C, and 1.17 × 10
−10
%
14
C, most of it in the
form of CO
2
. Because the atmosphere is well
mixed on short time scales, this relative
abundance is uniform around the globe -
although we note that, in the very short term, the
plumes from coal-fired power plants result in low
14
Tree-Ring Correlation
1481
0.4
0.2
P=0.26
0.0
-0.2
N=106 years
0.4
1489
0.2
P=0.14
0.0
N=114 years
-0.2
C content in the air, as all CO
2
from the power
plant is
14
C-dead (see Turnbull
et al.
, 2007). From
this atmospheric pool, the carbon in CO
2
is fixed
through photosynthesis in plants. Plants should,
therefore, be made of organic carbon that mimics
the isotopic ratio in the atmosphere during
growth, although the plant's ratio is offset from
that of the atmosphere due to fractionation dur-
ing photosynthesis. Once a plant or animal cell
dies, it no longer incorporates new carbon into
its organic material. Radioactive decay takes over
and begins to modify the ratio of the carbon iso-
topes in the cells. So, it is upon a cell's death that
the clock starts. We will see that the
14
C dating
technique is intertwined with at least two other
methods, U-Th and tree-ring dating, demonstrat-
ing how interdependent some of these techniques
have become.
Two principal means exist for measuring the
ratio of
14
C to
12
C in a sample.
Conventional
dat-
ing is done by counting the decays of
14
C to its
daughter
14
N through emission of an electron: a
beta (
β
) particle. The rate of decay, as repre-
sented by the beta particles, is commonly meas-
ured in decays per minute per gram of carbon
and requires samples on the order of a few grams
of carbon in size. For old samples, it is necessary
to count decays for long periods of time to record
enough decays for statistical reliability, and the
facilities in which such measurements are made
must be shielded from other radiation to avoid
miscounting. Even so, one can see easily from
the graph in Fig. 3.10 that the inevitable finite
errors associated with the isotopic measurement
grow in importance with sample age. For exam-
ple, we cannot distinguish between a sample of,
say, about 40 ka and one of infinite age. This
1480 1490 1500 1510
Calendar Year of Outermost Ring
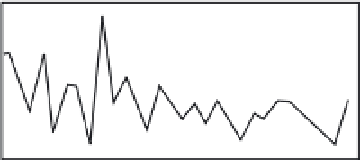
Fig. 3.9
Correlation of tree-width time series with
the master tree-ring time series as a function of
chosen start year.
Top series shows strongest correlation for a start date
(outermost ring) of calendar year 1481, whereas bottom
series is strongest for start date of calendar year 1489.
Correlation coefficients for all other start years have
probabilities of random occurrence that exceed the
labeled probability,
P
. Modified after Yamaguchi and
Hoblitt (1995).
longer ring-width time series to test against the
master chronology.
Tree-ring studies have also provided the basis
for calibration of the radiocarbon method (see
following section). The carbon from a particular
tree ring of known age (from counting back-
ward from the bark) can be
14
C dated using
recently developed techniques that permit dat-
ing of very small samples. Such calibration is
feasible for about the past 10 000 years, the
longest compiled tree-ring record.
Detailed studies of tree rings have allowed
geoscientists to place very precise dates on
important short-lived prehistoric geological
events, including rockslides, volcanic flows, and
even earthquakes. We will revisit this technique
in our discussion of prehistoric Cascadia
earthquakes.
Radiocarbon dating
The most commonly used technique to date
geomorphic features and surfaces that are less