Biomedical Engineering Reference
In-Depth Information
Creative phase
Specification
from 4.4
Generate
ideas
Idea selection
no
approve
File documentary evidence
in DHF
yes
Set project team
and milestones
Detailed design
no
yes
approve
no
Undertake design activities e.g.,
Design calculations
Dimension analysis
FMEA
Materials selection
QFD
Component selection
Risk analysis,
approve
Design drawings,
etc.
yes
Prototype
yes
no
approve
Evaluation
Figure 4.5
A typical detailed design procedure.
Evaluation
Select appropriate
team
Devise appropriate evaluation protocol,
e.g.,
Testing according to a required standard
Mechanical testing
Physical evaluation
Clinical evaluation
Pass/
Fail
Approve
Undertake evaluation
Report feedback
Figure 4.6
Design evaluation procedure.
4.5.5 Design Changes
This procedure is required to meet Section I and ISO 13485 7.3.7. In essence it has two main
purposes: firstly to ensure that any changes are made for the right reasons and undertaken
correctly; and secondly to ensure that the changes are made obvious so there can be no
mistake that a change has been made.



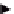


























































Search WWH ::

Custom Search