Biomedical Engineering Reference
In-Depth Information
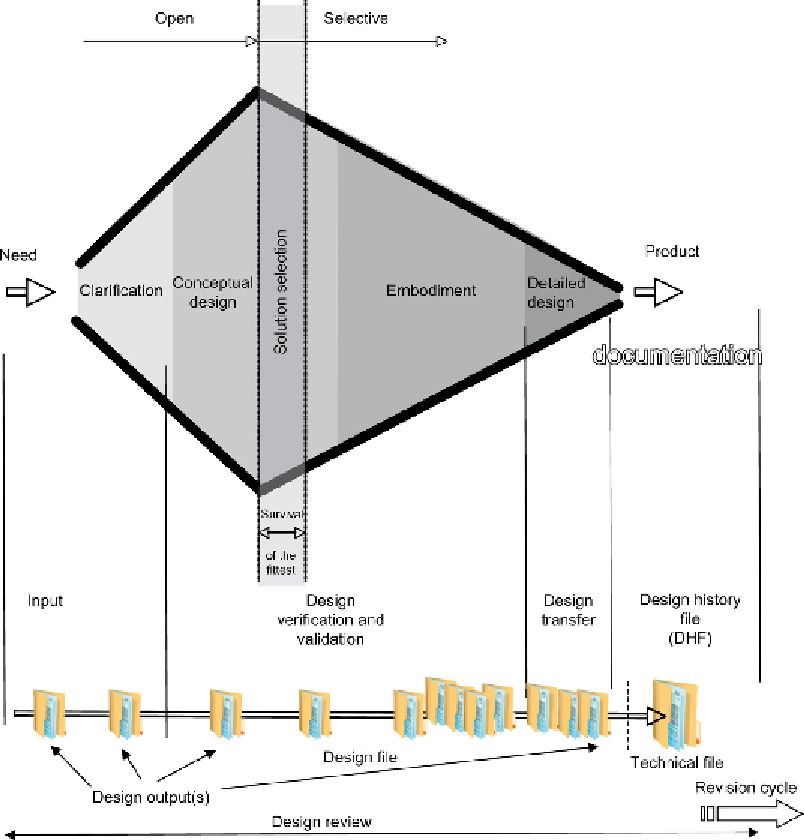
Figure 3.21
Divergent-convergent model mapped onto FDA guidelines.
the FDA guidelines or the EC guidelines as they use different languages. However, both refer
to ISO 9001 clause 4.4 (and subsequently its equivalent in ISO 13485). Better to use this as
the basis for your paper trail.
You will also note a similar example in the name of the design file. Once again, so long as
you cross-reference your design file, your technical file, or your design history file they will
Search WWH ::

Custom Search