Biomedical Engineering Reference
In-Depth Information
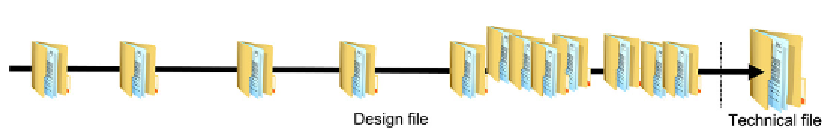
Figure 3.11
The document trail.
In comparison, where the early stages are fun and dynamic, the latter embodiment stage can
be seen to be monotonous. This is because we leave the creative element behind once we
start to converge. This phase, however, is highly critical as it is the stage where the important
decisions are made. In this phase we perform standard tasks and investigations to produce a
working design that meets the needs of the PDS. These can be repetitive, but hidden within
this monotony is the burningly obvious fact that every single device is actually a composite of
a mountain of smaller designs (remember the iceberg), each with their own particular need,
each needing a single solution to be selected, and each needing to be designed.
Figure 3.10
demonstrates this with numerous little divergent-convergent processes, each indicating their
own cog in the giant wheel of your overall project. This takes a lot of planning and project
management, and we will be looking at this later too.
All the way through the process you will need to produce a document trail.
Figure 3.11
illustrates this and demonstrates how the number of individual documents grows. These reside
within the
design file
. This is a “virtual document” as it is probably bigger than any file you
have on your shelf. It will probably be a collection of folders, and may even be a whole filing
cabinet. The important thing is that the design file records the whole of the design process
from start to finish. Every meeting, every decision, and every change must be recorded here.
We shall meet this again when we examine the
product approval process
. The whole process
culminates with the
technical file
. This is medical-devices-speak for the one document that
fully describes your device: how to make it; how it has been developed; how it has been
assessed; and how it meets any essential requirements.
Unless you follow a structured design
approach you will not be able to produce a technical file of sufficient rigor to get through any
medical devices audit.
This cannot be stressed enough - the applicable phrase is, “
Start as
you mean to go on.”
3.3 Managing Design
7
Do not assume that management of design is only a management of process. Design involves
people, so it is as much about people management as it is process management. It is beyond
the scope of this topic to turn you into a complete manager; however some simple tasks are
7
There are whole textbooks just on management of design - it is a subject in its own right. Universities have whole
master's degree programs on this topic.
Search WWH ::

Custom Search