Biomedical Engineering Reference
In-Depth Information
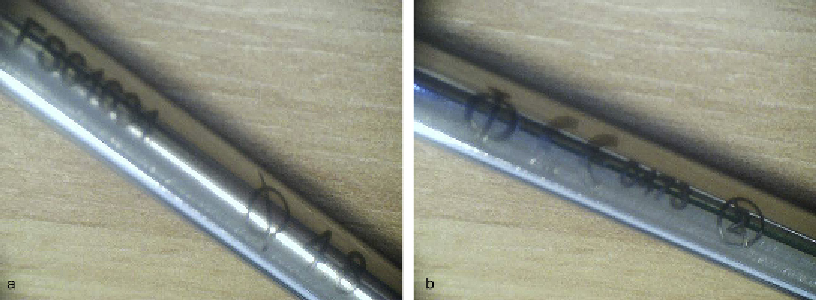
Figure 11.17
Example markings on an orthopedic bone drill: (a) CE mark and single use symbol; (b) lot number
and diameter. (
Courtesy Intelligent Orthopaedics Ltd
)
11.4.2 CE Mark
For all devices to be sold in the EC, a CE mark is required. For Class II devices and higher
this is the CE mark and the Notified Body's number, as in Section 11.2.1. Note: there is no
equivalent symbol for the USA.
11.4.3 Part Number and Lot Number
All items must permanently indicate their part number and their unique lot/batch number. The
main device should have an indication of the manufacturer permanently marked too (normally
the trademark symbol).
11.4.4 Size
For devices of specific sizes it is very common to mark the size in a recognizable form. For
devices that have to be a specific size to work this is essential.
11.5 IFUs and Surgical Techniques
This is often a bone of contention with people outside of medical devices. But the best way
to consider the differences between the two is to consider buying some domestic goods. If
you were buying a television then it would come with its own “instruction manual” - telling
you how to install it and use it - and you would probably make sure it is kept somewhere
safe (just in case). If, however, you were buying a pack of wood screws they would probably
come with some brief printed instruction sheet stating what they can be used for. In medical
devices we must always provide an IFU (instructions for use leaflet); however, depending
on the complexity of the system an instruction manual - or surgical technique (you pick the
appropriate title) - may be required.
Search WWH ::

Custom Search